Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [¹⁸F]DPA-714
- PMID: 22539835
- PMCID: PMC6703630
- DOI: 10.1523/JNEUROSCI.2900-11.2012
Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis rats by positron emission tomography using the mitochondrial 18 kDa translocator protein radioligand [¹⁸F]DPA-714
Abstract
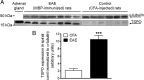
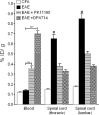
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the CNS. Activated microglia/macrophages play a key role in the immunopathogenesis of MS and its corresponding animal models, experimental autoimmune encephalomyelitis (EAE). Microglia activation begins at early stages of the disease and is associated with elevated expression of the 18 kDa mitochondrial translocator protein (TSPO). Thus, positron emission tomography (PET) imaging of microglial activation using TSPO-specific radioligands could be valuable for monitoring disease-associated neuroinflammatory processes. EAE was induced in rats using a fragment of myelin basic protein, yielding acute clinical disease that reflects extensive spinal cord inflammation. Enhanced TSPO expression in spinal cords of EAE rats versus those of controls was confirmed by Western blot and immunohistochemistry. Biodistribution studies in control and EAE rats were performed using the TSPO radioligand [¹⁸F]DPA-714 [N,N-diethyl-2-(2-(4-(2-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide]. At 1 h after injection, almost fivefold higher levels of [¹⁸F]DPA-714 were measured in spinal cords of EAE rats versus controls. The specific binding of [¹⁸F]DPA-714 to TSPO in spinal cords was confirmed in competition studies, using unlabeled (R,S)-PK11195 [(R,S)-N-methyl-N-(1-methylpropyl)-1-(2-chlorophenyl)isoquinoline-3-carboxamide)] or DPA-714 in excess. MicroPET studies affirm that this differential radioactivity uptake in spinal cords of EAE versus control rats could be detected and quantified. Using [¹⁸F]DPA-714, neuroinflammation in spinal cords of EAE-induced rats could be visualized by PET, offering a sensitive technique for monitoring neuroinflammatory lesions in the CNS and particularly in the spinal cord. In addition to current MRI protocols, this approach could provide molecular images of neuroinflammation for detection, monitoring, and research in MS.
Figures




References
-
- Agosta F, Absinta M, Sormani MP, Ghezzi A, Bertolotto A, Montanari E, Comi G, Filippi M. In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain. 2007;130:2211–2219. - PubMed
-
- Anholt RR, De Souza EB, Oster-Granite ML, Snyder SH. Peripheral-type benzodiazepine receptors: autoradiographic localization in whole-body sections of neonatal rats. J Pharmacol Exp Ther. 1985;233:517–526. - PubMed
-
- Banati RB. Visualising microglial activation in vivo. Glia. 2002;40:206–217. - PubMed
-
- Banati RB. Neuropathological imaging: in vivo detection of glial activation as a measure of disease and adaptive change in the brain. Br Med Bull. 2003;65:121–131. - PubMed
-
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123:2321–2337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
