Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock
- PMID: 22539850
- PMCID: PMC6703600
- DOI: 10.1523/JNEUROSCI.0469-12.2012
Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock
Abstract
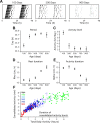
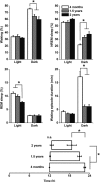
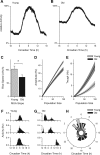
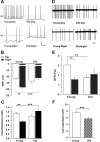
Aging is associated with a deterioration of daily (circadian) rhythms in physiology and behavior. Deficits in the function of the central circadian pacemaker in the suprachiasmatic nucleus (SCN) have been implicated, but the responsible mechanisms have not been clearly delineated. In this report, we characterize the progression of rhythm deterioration in mice to 900 d of age. Longitudinal behavioral and sleep-wake recordings in up to 30-month-old mice showed strong fragmentation of rhythms, starting at the age of 700 d. Patch-clamp recordings in this age group revealed deficits in membrane properties and GABAergic postsynaptic current amplitude. A selective loss of circadian modulation of fast delayed-rectifier and A-type K+ currents was observed. At the tissue level, phase synchrony of SCN neurons was grossly disturbed, with some subpopulations peaking in anti-phase and a reduction in amplitude of the overall multiunit activity rhythm. We propose that aberrant SCN rhythmicity in old animals--with electrophysiological arrhythmia at the single-cell level and phase desynchronization at the network level--can account for defective circadian function with aging.
Figures

References
-
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol. 2005;15:886–893. - PubMed
-
- Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. - PubMed
-
- Brown TM, Piggins HD. Electrophysiology of the suprachiasmatic circadian clock. Prog Neurobiol. 2007;82:229–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
