Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo
- PMID: 22540892
- PMCID: PMC3370420
- DOI: 10.1021/nn301282m
Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo
Abstract
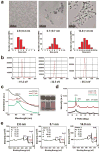
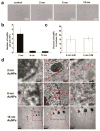
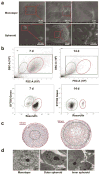
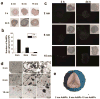
This work demonstrated that ultrasmall gold nanoparticles (AuNPs) smaller than 10 nm display unique advantages over nanoparticles larger than 10 nm in terms of localization to, and penetration of, breast cancer cells, multicellular tumor spheroids, and tumors in mice. Au@tiopronin nanoparticles that have tunable sizes from 2 to 15 nm with identical surface coatings of tiopronin and charge were successfully prepared. For monolayer cells, the smaller the Au@tiopronin NPs, the more AuNPs found in each cell. In addition, the accumulation of Au NPs in the ex vivo tumor model was size-dependent: smaller AuNPs were able to penetrate deeply into tumor spheroids, whereas 15 nm nanoparticles were not. Owing to their ultrasmall nanostructure, 2 and 6 nm nanoparticles showed high levels of accumulation in tumor tissue in mice after a single intravenous injection. Surprisingly, both 2 and 6 nm Au@tiopronin nanoparticles were distributed throughout the cytoplasm and nucleus of cancer cells in vitro and in vivo, whereas 15 nm Au@tiopronin nanoparticles were found only in the cytoplasm, where they formed aggregates. The ex vivo multicellular spheroid proved to be a good model to simulate in vivo tumor tissue and evaluate nanoparticle penetration behavior. This work gives important insights into the design and functionalization of nanoparticles to achieve high levels of accumulation in tumors.
Conflict of interest statement
Figures

Similar articles
-
Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors.Cancer Res. 2013 Jan 1;73(1):319-30. doi: 10.1158/0008-5472.CAN-12-2071. Epub 2012 Oct 16. Cancer Res. 2013. PMID: 23074284
-
Tumor penetration of Sub-10 nm nanoparticles: effect of dendrimer properties on their penetration in multicellular tumor spheroids.Nanomedicine. 2019 Oct;21:102059. doi: 10.1016/j.nano.2019.102059. Epub 2019 Jul 13. Nanomedicine. 2019. PMID: 31310808
-
Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy.Theranostics. 2020 Apr 6;10(12):5195-5208. doi: 10.7150/thno.45017. eCollection 2020. Theranostics. 2020. PMID: 32373207 Free PMC article.
-
Ultrasmall gold nanoparticles in cancer diagnosis and therapy.Theranostics. 2020 Mar 31;10(11):4944-4957. doi: 10.7150/thno.42471. eCollection 2020. Theranostics. 2020. PMID: 32308760 Free PMC article. Review.
-
Polymer decorated gold nanoparticles in nanomedicine conjugates.Adv Colloid Interface Sci. 2017 Nov;249:386-399. doi: 10.1016/j.cis.2017.01.007. Epub 2017 Feb 15. Adv Colloid Interface Sci. 2017. PMID: 28259207 Review.
Cited by
-
Nanoscale systems for local drug delivery.Nano Today. 2019 Oct;28:100765. doi: 10.1016/j.nantod.2019.100765. Epub 2019 Aug 26. Nano Today. 2019. PMID: 32831899 Free PMC article.
-
Identification of Zirconia Particle Uptake in Human Osteoblasts by ToF-SIMS Analysis and Particle-Size Effects on Cell Metabolism.Nanomaterials (Basel). 2022 Dec 1;12(23):4272. doi: 10.3390/nano12234272. Nanomaterials (Basel). 2022. PMID: 36500895 Free PMC article.
-
Gold nanoparticle-directed autophagy intervention for antitumor immunotherapy via inhibiting tumor-associated macrophage M2 polarization.Acta Pharm Sin B. 2022 Jul;12(7):3124-3138. doi: 10.1016/j.apsb.2022.02.008. Epub 2022 Feb 16. Acta Pharm Sin B. 2022. PMID: 35865102 Free PMC article.
-
Intercomparison of radiosensitization induced by gold and iron oxide nanoparticles in human glioblastoma cells irradiated by 6 MV photons.Sci Rep. 2022 Jun 10;12(1):9602. doi: 10.1038/s41598-022-13368-x. Sci Rep. 2022. PMID: 35688846 Free PMC article.
-
High Loading of Hydrophobic and Hydrophilic Agents via Small Immunostimulatory Carrier for Enhanced Tumor Penetration and Combinational Therapy.Theranostics. 2020 Jan 1;10(3):1136-1150. doi: 10.7150/thno.38287. eCollection 2020. Theranostics. 2020. PMID: 31938056 Free PMC article.
References
-
- Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, Hawkins M, O’Shaughnessy J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil-Based Paclitaxel in Women with Breast Cancer. J Clin Oncol. 2005;23:7794–7803. - PubMed
-
- Torchilin VP. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat Rev Drug Discovery. 2005;4:145–160. - PubMed
-
- Minchinton AI, Tannock IF. Drug Penetration in Solid Tumours. Nat Rev Cancer. 2006;6:583–592. - PubMed
-
- Mikhail AS, Allen C. Block Copolymer Micelles for Delivery of Cancer Therapy: Transport at the Whole Body, Tissue and Cellular Levels. J Controlled Release. 2009;138:214–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

