A clinical trial to maintain glycemic control in youth with type 2 diabetes
- PMID: 22540912
- PMCID: PMC3478667
- DOI: 10.1056/NEJMoa1109333
A clinical trial to maintain glycemic control in youth with type 2 diabetes
Abstract
Background: Despite the increasing prevalence of type 2 diabetes in youth, there are few data to guide treatment. We compared the efficacy of three treatment regimens to achieve durable glycemic control in children and adolescents with recent-onset type 2 diabetes.
Methods: Eligible patients 10 to 17 years of age were treated with metformin (at a dose of 1000 mg twice daily) to attain a glycated hemoglobin level of less than 8% and were randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg twice a day) or a lifestyle-intervention program focusing on weight loss through eating and activity behaviors. The primary outcome was loss of glycemic control, defined as a glycated hemoglobin level of at least 8% for 6 months or sustained metabolic decompensation requiring insulin.
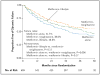
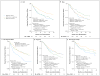
Results: Of the 699 randomly assigned participants (mean duration of diagnosed type 2 diabetes, 7.8 months), 319 (45.6%) reached the primary outcome over an average follow-up of 3.86 years. Rates of failure were 51.7% (120 of 232 participants), 38.6% (90 of 233), and 46.6% (109 of 234) for metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle intervention, respectively. Metformin plus rosiglitazone was superior to metformin alone (P=0.006); metformin plus lifestyle intervention was intermediate but not significantly different from metformin alone or metformin plus rosiglitazone. Prespecified analyses according to sex and race or ethnic group showed differences in sustained effectiveness, with metformin alone least effective in non-Hispanic black participants and metformin plus rosiglitazone most effective in girls. Serious adverse events were reported in 19.2% of participants.
Conclusions: Monotherapy with metformin was associated with durable glycemic control in approximately half of children and adolescents with type 2 diabetes. The addition of rosiglitazone, but not an intensive lifestyle intervention, was superior to metformin alone. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; TODAY ClinicalTrials.gov number, NCT00081328.).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
TODAY--a stark glimpse of tomorrow.N Engl J Med. 2012 Jun 14;366(24):2315-6. doi: 10.1056/NEJMe1204710. Epub 2012 Apr 29. N Engl J Med. 2012. PMID: 22540913 No abstract available.
-
Diabetes: early initiation of intensive therapy might benefit children with type 2 diabetes mellitus.Nat Rev Endocrinol. 2012 May 22;8(7):382. doi: 10.1038/nrendo.2012.77. Nat Rev Endocrinol. 2012. PMID: 22614716 No abstract available.
-
Glycemic control in youth with type 2 diabetes.N Engl J Med. 2012 Sep 13;367(11):1066; author reply 1066-7. doi: 10.1056/NEJMc1208408. N Engl J Med. 2012. PMID: 22970956 No abstract available.
-
[A clinical trial to maintain glycemic control in youth with type 2 diabetes].Rev Clin Esp. 2012 Nov;212(10):500. doi: 10.1016/j.rce.2012.07.015. Rev Clin Esp. 2012. PMID: 23289101 Spanish. No abstract available.
-
Update in endocrinology: evidence published in 2012.Ann Intern Med. 2013 Jun 4;158(11):821-4. doi: 10.7326/0003-4819-158-11-201306040-00106. Ann Intern Med. 2013. PMID: 23580066 No abstract available.
Comment on
-
Treating type 2 diabetes in youth: a depressing picture.J R Coll Physicians Edinb. 2012;42(3):228. doi: 10.4997/JRCPE.2012.309. J R Coll Physicians Edinb. 2012. PMID: 22953318 Clinical Trial.
References
-
- Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. - PubMed
-
- Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–72. - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. - PubMed
-
- Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124:136–45. - PubMed
-
- Pinhas-Hamiel O, Standiford D, Hamiel D, Dolan LM, Cohen RM, Zeitler PS. The type 2 family: a setting for development and treatment of adolescent type 2 diabetes mellitus. Arch Pediatr Adolesc Med. 1999;153:1063–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1-RR024153/RR/NCRR NIH HHS/United States
- U01-DK61239/DK/NIDDK NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- U01 DK061212/DK/NIDDK NIH HHS/United States
- M01 RR014467/RR/NCRR NIH HHS/United States
- U01 DK061230/DK/NIDDK NIH HHS/United States
- M01-RR00036/RR/NCRR NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01 RR000084/RR/NCRR NIH HHS/United States
- M01-RR00043-45/RR/NCRR NIH HHS/United States
- U01 DK061254/DK/NIDDK NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- UL1 RR024989/RR/NCRR NIH HHS/United States
- P30 DK092950/DK/NIDDK NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- M01-RR00125/RR/NCRR NIH HHS/United States
- U01-DK61242/DK/NIDDK NIH HHS/United States
- M01 RR000069/RR/NCRR NIH HHS/United States
- U01-DK61254/DK/NIDDK NIH HHS/United States
- U01-DK61212/DK/NIDDK NIH HHS/United States
- UL1-RR025758/RR/NCRR NIH HHS/United States
- UL1-RR024989/RR/NCRR NIH HHS/United States
- U01 DK061242/DK/NIDDK NIH HHS/United States
- U01-DK61230/DK/NIDDK NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- M01-RR01066/RR/NCRR NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- UL1-RR024139/RR/NCRR NIH HHS/United States
- UL1-RR025780/RR/NCRR NIH HHS/United States
- UL1-RR024992/RR/NCRR NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- UL1-RR024134/RR/NCRR NIH HHS/United States
- M01-RR00069/RR/NCRR NIH HHS/United States
- M01-RR14467/RR/NCRR NIH HHS/United States
- M01 RR000125/RR/NCRR NIH HHS/United States
- U01 DK061239/DK/NIDDK NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- M01-RR00084/RR/NCRR NIH HHS/United States
- UL1 TR000130/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
