The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients
- PMID: 22540992
- PMCID: PMC3446469
- DOI: 10.1186/ar3819
The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients
Abstract
Introduction: B cell depletion therapy is efficacious in rheumatoid arthritis (RA) patients failing on tumor necrosis factor (TNF) blocking agents. However, approximately 40% to 50% of rituximab (RTX) treated RA patients have a poor response. We investigated whether baseline gene expression levels can discriminate between clinical non-responders and responders to RTX.
Methods: In 14 consecutive RA patients starting on RTX (test cohort), gene expression profiling on whole peripheral blood RNA was performed by Illumina® HumanHT beadchip microarrays. Supervised cluster analysis was used to identify genes expressed differentially at baseline between responders and non-responders based on both a difference in 28 joints disease activity score (ΔDAS28 < 1.2) and European League against Rheumatism (EULAR) response criteria after six months RTX. Genes of interest were measured by quantitative real-time PCR and tested for their predictive value using receiver operating characteristics (ROC) curves in an independent validation cohort (n = 26).
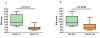
Results: Genome-wide microarray analysis revealed a marked variation in the peripheral blood cells between RA patients before the start of RTX treatment. Here, we demonstrated that only a cluster consisting of interferon (IFN) type I network genes, represented by a set of IFN type I response genes (IRGs), that is, LY6E, HERC5, IFI44L, ISG15, MxA, MxB, EPSTI1 and RSAD2, was associated with ΔDAS28 and EULAR response outcome (P = 0.0074 and P = 0.0599, respectively). Based on the eight IRGs an IFN-score was calculated that reached an area under the curve (AUC) of 0.82 to separate non-responders from responders in an independent validation cohort of 26 patients using Receiver Operator Characteristics (ROC) curves analysis according to ΔDAS28 < 1.2 criteria. Advanced classifier analysis yielded a three IRG-set that reached an AUC of 87%. Comparable findings applied to EULAR non-response criteria.
Conclusions: This study demonstrates clinical utility for the use of baseline IRG expression levels as a predictive biomarker for non-response to RTX in RA.
Figures


References
-
- Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. - DOI - PubMed
-
- Rantapää-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

