Neurological phenotypes for Down syndrome across the life span
- PMID: 22541290
- PMCID: PMC3417824
- DOI: 10.1016/B978-0-444-54299-1.00006-6
Neurological phenotypes for Down syndrome across the life span
Abstract
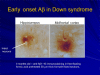
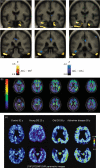
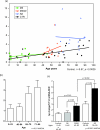
This chapter reviews the neurological phenotype of Down syndrome (DS) in early development, childhood, and aging. Neuroanatomic abnormalities in DS are manifested as aberrations in gross brain structure as well as characteristic microdysgenetic changes. As the result of these morphological abnormalities, brain circuitry is impaired. While an intellectual disability is ubiquitous in DS, there is a wide range of variation in cognitive performance and a growing understanding between aberrant brain circuitry and the cognitive phenotype. Hypotonia is most marked at birth, affecting gait and ligamentous laxity. Seizures are bimodal in presentation with infantile spasms common in infancy and generalized seizures associated with cognitive decline observed in later years. While all individuals have the characteristic neuropathology of Alzheimer's disease (AD) by age 40 years, the prevalence of dementia is not universal. The tendency to develop AD is related, in part, to several genes on chromosome 21 that are overexpressed in DS. Intraneuronal accumulation of β-amyloid appears to trigger a cascade of neurodegeneration resulting in the neuropathological and clinical manifestations of dementia. Functional brain imaging has elucidated the temporal sequence of amyloid deposition and glucose metabolic rate in the development of dementia in DS. Mitochondrial abnormalities contribute to oxidative stress which is part of AD pathogenesis in DS as well as AD in the general population. A variety of medical comorbidities threaten cognitive performance including sleep apnea, abnormalities in thyroid metabolism, and behavioral disturbances. Mouse models for DS are providing a platform for the formulation of clinical trials with intervention targeted to synaptic plasticity, brain biochemistry, and morphological brain alterations.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
-
- Agiovlasitis S, McCubbin JA, Yun J, Pavol MJ, Widrick JJ. Economy and preferred speed of walking in adults with and without Down syndrome. Adapted Physical Activity Quarterly. 2009;26:118–130. - PubMed
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neurosciences. 2008;31:137–145. - PubMed
-
- Andrade DM. Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain. Human Genetics. 2009;126:173–193. - PubMed
-
- Andreou G, Galanopoulou C, Gourgoulianis K, Karapetsas A, Molyvdas P. Cognitive status in Down syndrome individuals with sleep disordered breathing deficits (SDB). Brain and Cognition. 2002;50:145–149. - PubMed
-
- Andriolo RB, El Dib RP, Ramos L, Atallah AN, da Silva EM. Aerobic exercise training programmes for improving physical and psychosocial health in adults with Down syndrome. Cochrane Database of Systematic Reviews. 2010:CD005176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

