Localized products of futile cycle/lrmp promote centrosome-nucleus attachment in the zebrafish zygote
- PMID: 22542100
- PMCID: PMC3360835
- DOI: 10.1016/j.cub.2012.03.058
Localized products of futile cycle/lrmp promote centrosome-nucleus attachment in the zebrafish zygote
Abstract
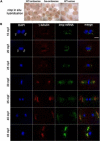
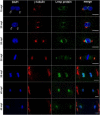
Background: The centrosome has a well-established role as a microtubule organizer during mitosis and cytokinesis. In addition, it facilitates the union of parental haploid genomes following fertilization by nucleating a microtubule aster along which the female pronucleus migrates toward the male pronucleus. Stable associations between the sperm aster and the pronuclei are essential during this directed movement.
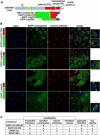
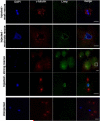
Results: Our studies reveal that the zebrafish gene futile cycle (fue) is required in the zygote for male pronucleus-centrosome attachment and female pronuclear migration. We show that fue encodes a novel, maternally-provided long form of lymphoid-restricted membrane protein (lrmp), a vertebrate-specific gene of unknown function. Both maternal lrmp messenger RNA (mRNA) and protein are highly localized in the zygote, in a largely overlapping pattern at nuclear membranes, centrosomes, and spindles. Truncated Lrmp-EGFP fusion proteins identified subcellular targeting signals in the C terminus of Lrmp; however, endogenous mRNA localization is likely important to ensure strict spatial expression of the protein. Localization of both Lrmp protein and lrmp RNA is defective in fue mutant embryos, indicating that correct targeting of lrmp gene products is dependent on Lrmp function.
Conclusions: Lrmp is a conserved vertebrate gene whose maternally inherited products are essential for nucleus-centrosome attachment and pronuclear congression during fertilization. Precise subcellular localization of lrmp products also suggests a requirement for strict spatiotemporal regulation of their function in the early embryo.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Pronuclear migration: no attachment? No union, but a futile cycle!Curr Biol. 2012 May 22;22(10):R409-11. doi: 10.1016/j.cub.2012.03.062. Curr Biol. 2012. PMID: 22625860
References
-
- Chambers EL. The movement of the egg nucleus in relation to the sperm aster in the echinoderm egg. J. Exp. Biol. 1939;16:409–424.
-
- Navara CS, First NL, Schatten G. Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev. Biol. 1994;162:29–40. - PubMed
-
- Reinsch S, Gonczy P. Mechanisms of nuclear positioning. J. Cell. Sci. 1998;111:2283–2295. - PubMed
-
- Delattre M, Gonczy P. The arithmetic of centrosome biogenesis. J. Cell. Sci. 2004;117:1619–1630. - PubMed
-
- Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev. Biol. 1994;165:299–335. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

