Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates
- PMID: 22542104
- PMCID: PMC3361572
- DOI: 10.1016/j.chembiol.2012.03.009
Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates
Abstract
The endocannabinoids 2-arachidonoyl glycerol (2-AG) and N-arachidonoyl ethanolamine (anandamide) are principally degraded by monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH), respectively. The recent discovery of O-aryl carbamates such as JZL184 as selective MAGL inhibitors has enabled functional investigation of 2-AG signaling pathways in vivo. Nonetheless, JZL184 and other reported MAGL inhibitors still display low-level cross-reactivity with FAAH and peripheral carboxylesterases, which can complicate their use in certain biological studies. Here, we report a distinct class of O-hexafluoroisopropyl (HFIP) carbamates that inhibits MAGL in vitro and in vivo with excellent potency and greatly improved selectivity, including showing no detectable cross-reactivity with FAAH. These findings designate HFIP carbamates as a versatile chemotype for inhibiting MAGL and should encourage the pursuit of other serine hydrolase inhibitors that bear reactive groups resembling the structures of natural substrates.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

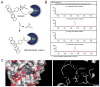

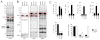

Comment in
-
Discrimination between two endocannabinoids.Chem Biol. 2012 May 25;19(5):545-7. doi: 10.1016/j.chembiol.2012.05.001. Chem Biol. 2012. PMID: 22633404 No abstract available.
References
-
- Bertrand T, Augé F, Houtmann J, Rak A, Vallée F, Mikol V, Berne PF, Michot N, Cheuret D, Hoornaert C, et al. Structural basis for human monoglyceride lipase inhibition. J Mol Biol. 2010;396:663–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

