On the perception of probable things: neural substrates of associative memory, imagery, and perception
- PMID: 22542178
- PMCID: PMC3361508
- DOI: 10.1016/j.neuron.2012.04.001
On the perception of probable things: neural substrates of associative memory, imagery, and perception
Abstract
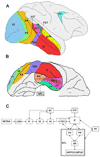
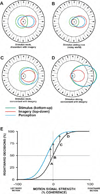
Perception is influenced both by the immediate pattern of sensory inputs and by memories acquired through prior experiences with the world. Throughout much of its illustrious history, however, study of the cellular basis of perception has focused on neuronal structures and events that underlie the detection and discrimination of sensory stimuli. Relatively little attention has been paid to the means by which memories interact with incoming sensory signals. Building upon recent neurophysiological/behavioral studies of the cortical substrates of visual associative memory, I propose a specific functional process by which stored information about the world supplements sensory inputs to yield neuronal signals that can account for visual perceptual experience. This perspective represents a significant shift in the way we think about the cellular bases of perception.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures






References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. (A) 1985;2:284–299. - PubMed
-
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 1984;52:1106–1130. - PubMed
-
- Albright TD. Cortical processing of visual motion. In: Wallman J, Miles FA, editors. Visual Motion and its Use in the Stabilization of Gaze. Amsterdam: Elsevier; 1993. pp. 177–201.
-
- Albright TD. 'My most true mind thus makes mine eye untrue'. Trends Neurosci. 1995;18:331–333. - PubMed
-
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J. Neurophysiol. 1984;51:16–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

