Dynactin is required for transport initiation from the distal axon
- PMID: 22542186
- PMCID: PMC3347924
- DOI: 10.1016/j.neuron.2012.02.025
Dynactin is required for transport initiation from the distal axon
Abstract
Dynactin is a required cofactor for the minus-end-directed microtubule motor cytoplasmic dynein. Mutations within the highly conserved CAP-Gly domain of dynactin cause neurodegenerative disease. Here, we show that the CAP-Gly domain is necessary to enrich dynactin at the distal end of primary neurons. While the CAP-Gly domain is not required for sustained transport along the axon, we find that the distal accumulation facilitates the efficient initiation of retrograde vesicular transport from the neurite tip. Neurodegenerative disease mutations in the CAP-Gly domain prevent the distal enrichment of dynactin thereby inhibiting the initiation of retrograde transport. Thus, we propose a model in which distal dynactin is a key mediator in promoting the interaction among the microtubule, dynein motor, and cargo for the efficient initiation of transport. Mutations in the CAP-Gly domain disrupt the formation of the motor-cargo complex, highlighting the specific defects in axonal transport that may lead to neurodegeneration.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
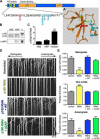
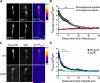
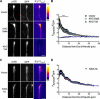
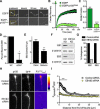



Comment in
-
The CAP-Gly of p150: one domain, two diseases, and a function at the end.Neuron. 2012 Apr 26;74(2):211-3. doi: 10.1016/j.neuron.2012.04.003. Neuron. 2012. PMID: 22542174 Free PMC article.
References
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol. 2008;9:309–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

