Preparation of porous polymer monoliths featuring enhanced surface coverage with gold nanoparticles
- PMID: 22542442
- PMCID: PMC3424317
- DOI: 10.1016/j.chroma.2012.04.007
Preparation of porous polymer monoliths featuring enhanced surface coverage with gold nanoparticles
Abstract
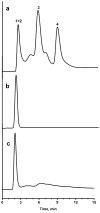
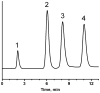
A new approach to the preparation of porous polymer monoliths with enhanced coverage of pore surface with gold nanoparticles has been developed. First, a generic poly(glycidyl methacrylate-co-ethylene dimethacrylate) monolith was reacted with cystamine followed by the cleavage of its disulfide bonds with tris(2-carboxylethyl)phosphine, which liberated the desired thiol groups. Dispersions of gold nanoparticles with sizes varying from 5 to 40 nm were then pumped through the functionalized monoliths. The materials were then analyzed using both energy dispersive X-ray spectroscopy and thermogravimetric analysis. We found that the quantity of attached gold was dependent on the size of nanoparticles, with the maximum attachment of more than 60 wt% being achieved with 40 nm nanoparticles. Scanning electron micrographs of the cross sections of all the monoliths revealed the formation of a non-aggregated, homogenous monolayer of nanoparticles. The surface of the bound gold was functionalized with 1-octanethiol and 1-octadecanethiol, and these monolithic columns were used successfully for the separations of proteins in reversed phase mode. The best separations were obtained using monoliths modified with 15, 20, and 30 nm nanoparticles since these sizes produced the most dense coverage of pore surface with gold.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures







References
-
- Hjertén S, Liao JL, Zhang R. J Chromatogr. 1989;473:273.
-
- Tennikova TB, Svec F, Belenkii BG. J Liquid Chromatogr. 1990;13:63.
-
- Svec F, Fréchet JMJ. Anal Chem. 1992;54:820.
-
- Svec F, Fréchet JMJ. Science. 1996;273:205. - PubMed
-
- Minakuchi H, Nakanishi K, Soga N, Ishizuka N, Tanaka N. Anal Chem. 1996;68:3498. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

