The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation
- PMID: 22542597
- PMCID: PMC3388946
- DOI: 10.1016/j.ydbio.2012.03.026
The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation
Abstract
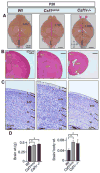
The CSF-1 receptor (CSF-1R) regulates CNS microglial development. However, the localization and developmental roles of this receptor and its ligands, IL-34 and CSF-1, in the brain are poorly understood. Here we show that compared to wild type mice, CSF-1R-deficient (Csf1r-/-) mice have smaller brains of greater mass. They further exhibit an expansion of lateral ventricle size, an atrophy of the olfactory bulb and a failure of midline crossing of callosal axons. In brain, IL-34 exhibited a broader regional expression than CSF-1, mostly without overlap. Expression of IL-34, CSF-1 and the CSF-1R were maximal during early postnatal development. However, in contrast to the expression of its ligands, CSF-1R expression was very low in adult brain. Postnatal neocortical expression showed that CSF-1 was expressed in layer VI, whereas IL-34 was expressed in the meninges and layers II-V. The broader expression of IL-34 is consistent with its previously implicated role in microglial development. The differential expression of CSF-1R ligands, with respect to CSF-1R expression, could reflect their CSF-1R-independent signaling. Csf1r-/- mice displayed increased proliferation and apoptosis of neocortical progenitors and reduced differentiation of specific excitatory neuronal subtypes. Indeed, addition of CSF-1 or IL-34 to microglia-free, CSF-1R-expressing dorsal forebrain clonal cultures, suppressed progenitor self-renewal and enhanced neuronal differentiation. Consistent with a neural developmental role for the CSF-1R, ablation of the Csf1r gene in Nestin-positive neural progenitors led to a smaller brain size, an expanded neural progenitor pool and elevated cellular apoptosis in cortical forebrain. Thus our results also indicate novel roles for the CSF-1R in the regulation of corticogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

