Clinical decision support with automated text processing for cervical cancer screening
- PMID: 22542812
- PMCID: PMC3422840
- DOI: 10.1136/amiajnl-2012-000820
Clinical decision support with automated text processing for cervical cancer screening
Abstract
Objective: To develop a computerized clinical decision support system (CDSS) for cervical cancer screening that can interpret free-text Papanicolaou (Pap) reports.
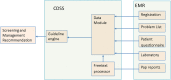
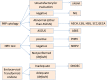
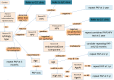
Materials and methods: The CDSS was constituted by two rulebases: the free-text rulebase for interpreting Pap reports and a guideline rulebase. The free-text rulebase was developed by analyzing a corpus of 49 293 Pap reports. The guideline rulebase was constructed using national cervical cancer screening guidelines. The CDSS accesses the electronic medical record (EMR) system to generate patient-specific recommendations. For evaluation, the screening recommendations made by the CDSS for 74 patients were reviewed by a physician.
Results and discussion: Evaluation revealed that the CDSS outputs the optimal screening recommendations for 73 out of 74 test patients and it identified two cases for gynecology referral that were missed by the physician. The CDSS aided the physician to amend recommendations in six cases. The failure case was because human papillomavirus (HPV) testing was sometimes performed separately from the Pap test and these results were reported by a laboratory system that was not queried by the CDSS. Subsequently, the CDSS was upgraded to look up the HPV results missed earlier and it generated the optimal recommendations for all 74 test cases.
Limitations: Single institution and single expert study.
Conclusion: An accurate CDSS system could be constructed for cervical cancer screening given the standardized reporting of Pap tests and the availability of explicit guidelines. Overall, the study demonstrates that free text in the EMR can be effectively utilized through natural language processing to develop clinical decision support tools.
Conflict of interest statement
Figures
References
-
- Yabroff KR, Saraiya M, Meissner HI, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: a national survey, 2006 to 2007. Ann Intern Med 2009;151:602–11 - PubMed
-
- Saraiya M, Berkowitz Z, Yabroff KR, et al. Cervical cancer screening with both human papillomavirus and papanicolaou testing vs papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med 2010;170:977–85 - PubMed
-
- Lee JW, Berkowitz Z, Saraiya M. Low-risk human papillomavirus testing and other non recommended human papillomavirus testing practices among U.S. health care providers. Obstet Gynecol 2011;118:4–13 - PubMed
-
- Roland KB, Soman A, Benard VB, et al. Human papillomavirus and papanicolaou tests screening interval recommendations in the United States. Am J Obstet Gynecol 2011;205:447e1–8. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

