LTB4 is a signal-relay molecule during neutrophil chemotaxis
- PMID: 22542839
- PMCID: PMC4141281
- DOI: 10.1016/j.devcel.2012.02.003
LTB4 is a signal-relay molecule during neutrophil chemotaxis
Abstract
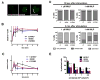
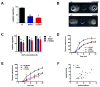
Neutrophil recruitment to inflammation sites purportedly depends on sequential waves of chemoattractants. Current models propose that leukotriene B(4) (LTB(4)), a secondary chemoattractant secreted by neutrophils in response to primary chemoattractants such as formyl peptides, is important in initiating the inflammation process. In this study we demonstrate that LTB(4) plays a central role in neutrophil activation and migration to formyl peptides. We show that LTB(4) production dramatically amplifies formyl peptide-mediated neutrophil polarization and chemotaxis by regulating specific signaling pathways acting upstream of actin polymerization and MyoII phosphorylation. Importantly, by analyzing the migration of neutrophils isolated from wild-type mice and mice lacking the formyl peptide receptor 1, we demonstrate that LTB(4) acts as a signal to relay information from cell to cell over long distances. Together, our findings imply that LTB(4) is a signal-relay molecule that exquisitely regulates neutrophil chemotaxis to formyl peptides, which are produced at the core of inflammation sites.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. - PubMed
-
- Berger M, Budhu S, Lu E, Li Y, Loike D, Silverstein SC, Loike JD. Different G(i)-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol. 2002;71:798–806. - PubMed
-
- Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. - PubMed
-
- Canetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171:1009–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

