E-cadherin promotes proliferation of human ovarian cancer cells in vitro via activating MEK/ERK pathway
- PMID: 22543706
- PMCID: PMC4010376
- DOI: 10.1038/aps.2012.30
E-cadherin promotes proliferation of human ovarian cancer cells in vitro via activating MEK/ERK pathway
Abstract
Aim: E-cadherin is unusually highly expressed in most ovarian cancers. This study was designed to investigate the roles of E-cadherin in the carcinogenesis and progression of ovarian cancers.
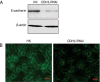
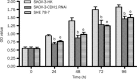
Methods: Human ovarian adenocarcinoma cell line SKOV-3 was examined. E-cadherin gene CDH1 in SKOV-3 cells was knocked down via RNA interference (RNAi), and the resultant variation of biological behavior was observed using CCK-8 and colony formation experiment. E-cadherin-mediated Ca(2+)-dependent cell-cell adhesion was used to study the mechanisms underlying the effects of E-cadherin on the proliferation and survival of SKOV-3 cells. The expression levels of E-cadherin, extracellular signal-related kinase (ERK), phosphorylated ERK (P-ERK) were measured using Western blot assays.
Results: Transfection with CDH1-siRNA for 24-96 h significantly suppressed the growth and proliferation of SKOV-3 cells. E-cadherin-mediated calcium-dependent cell-cell adhesion of SKOV-3 cells resulted in a rapid increase of P-ERK, but did not modify the expression of ERK protein. The phosphorylation of ERK in the cells was blocked by pretreatment with the MEK1 specific inhibitor PD98059 (50 μmol/L), but not by the PI3K inhibitor wortmannin (1 μmol/L) or PKA inhibitor H89 (10 μmol/L).
Conclusion: E-cadherin may function as a tumor proliferation enhancer via activating the MEK/ERK pathway in development of ovarian epithelial cancers.
Figures


References
-
- Fang K, Mukhopadhyay T, Shih SH. Retinoic acid modulates epidermal growth factor receptor expression in human lung epithelial cancer cells. J Biomed Sci. 1995;2:256–62. - PubMed
-
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–21. - PubMed
-
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–5. - PubMed
-
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

