The regulation of N-terminal Huntingtin (Htt552) accumulation by Beclin1
- PMID: 22543707
- PMCID: PMC4010368
- DOI: 10.1038/aps.2012.14
The regulation of N-terminal Huntingtin (Htt552) accumulation by Beclin1
Abstract
Aim: Huntingtin protein (Htt) was a neuropathological hallmark in human Huntington's Disease. The study aimed to investigate whether the macroautophagy regulator, Beclin1, was involved in the degradation of Htt.
Methods: PC12 cells and primary cultured brain neurons of rats were examined. pDC316 adenovirus shuttle plasmid was used to mediate the expression of wild-type Htt-18Q-552 or mutant Htt-100Q-552 in PC12 cells. The expression of the autophagy-related proteins LC3 II and Beclin1, as well as the lysosome-associated enzymes Cathepsin B and L was evaluated using Western blotting. The locations of Beclin1 and Htt were observed with immunofluorescence and confocal microscope.
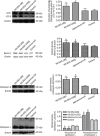
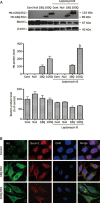
Results: Htt552 expression increased the expression of LC3 II, Beclin1, cathepsin B and L in autophagy/lysosomal degradation pathway. Treatment with the autophagy inhibitor 3-MA or the proteasome inhibitors lactacystin and MG-132 increased Htt552 levels in PC12 cells infected with Ad-Htt-18Q-552 or Ad-Htt-100Q-552. The proteasome inhibitor caused a higher accumulation of Htt552-18Q than Htt552-100Q, and the autophagy inhibitor resulted in a higher accumulation of Htt552-100Q than Htt552-18Q. Similar results were observed in primary cultured neurons infected with adenovirus. In Htt552-expressing cells, Beclin1 was redistributed from the nucleus to the cytoplasm. Htt siRNA prevented Beclin1 redistribution in starvation conditions. Blockade of Beclin1 nuclear export by leptomycin B or Beclin1 deficiency caused by RNA interference induced the formation of mHtt552 aggregates.
Conclusion: Beclin1 regulates the accumulation of Htt via macroautophagy.
Figures





References
-
- Kim YJ, Yi Y, Sapp E, Wang Y, Cuiffo B, Kegel KB, et al. Caspase 3-cleaved N-terminal fragments of wild-type and mutant Htt are present in normal and Huntington's disease brains, associate with membranes, and undergo calpain-dependent proteolysis. Proc Natl Acad Sci USA. 2001;98:12784–9. - PMC - PubMed
-
- Qin ZH, Gu ZL, Lin F. The advancement of molecular pathology of Huntington's disease. Chin Pham Bull. 2004;20:378–82.
-
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–38. - PubMed
-
- Nasir J, Floresco SB, O'Kusky JR, Diewert VM, Richman JM, Zeisler J, et al. Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995;81:811–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

