Implications of microfauna-host interactions for trypanosome transmission dynamics in Glossina fuscipes fuscipes in Uganda
- PMID: 22544247
- PMCID: PMC3370491
- DOI: 10.1128/AEM.00806-12
Implications of microfauna-host interactions for trypanosome transmission dynamics in Glossina fuscipes fuscipes in Uganda
Abstract
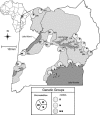
Tsetse flies (Diptera: Glossinidae) are vectors for African trypanosomes (Euglenozoa: kinetoplastida), protozoan parasites that cause African trypanosomiasis in humans (HAT) and nagana in livestock. In addition to trypanosomes, two symbiotic bacteria (Wigglesworthia glossinidia and Sodalis glossinidius) and two parasitic microbes, Wolbachia and a salivary gland hypertrophy virus (SGHV), have been described in tsetse. Here we determined the prevalence of and coinfection dynamics between Wolbachia, trypanosomes, and SGHV in Glossina fuscipes fuscipes in Uganda over a large geographical scale spanning the range of host genetic and spatial diversity. Using a multivariate analysis approach, we uncovered complex coinfection dynamics between the pathogens and statistically significant associations between host genetic groups and pathogen prevalence. It is important to note that these coinfection dynamics and associations with the host were not apparent by univariate analysis. These associations between host genotype and pathogen are particularly evident for Wolbachia and SGHV where host groups are inversely correlated for Wolbachia and SGHV prevalence. On the other hand, trypanosome infection prevalence is more complex and covaries with the presence of the other two pathogens, highlighting the importance of examining multiple pathogens simultaneously before making generalizations about infection and spatial patterns. It is imperative to note that these novel findings would have been missed if we had employed the standard univariate analysis used in previous studies. Our results are discussed in the context of disease epidemiology and vector control.
Figures




References
-
- Abd-Alla A, et al. 2007. Development of a non-destructive PCR method for detection of the salivary gland hypertrophy virus (SGHV) in tsetse flies. J. Virol. Methods 139:143–149 - PubMed
-
- Abd-Alla AM, et al. 2009. Quantitative PCR analysis of the salivary gland hypertrophy virus (GpSGHV) in a laboratory colony of Glossina pallidipes. Virus Res. 139:48–53 - PubMed
-
- Abd-Alla AM, et al. 2010. Dynamics of the salivary gland hypertrophy virus in laboratory colonies of Glossina pallidipes (Diptera: Glossinidae). Virus Res. 150:103–110 - PubMed
-
- Abila PP, et al. 2008. High levels of genetic differentiation between Ugandan Glossina fuscipes fuscipes populations separated by Lake Kyoga. PLoS Negl. Trop. Dis. 2:e242 doi:10.1371/journal.pntd.0000242 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

