IKKα-mediated signaling circuitry regulates early B lymphopoiesis during hematopoiesis
- PMID: 22544702
- PMCID: PMC3369682
- DOI: 10.1182/blood-2012-01-401547
IKKα-mediated signaling circuitry regulates early B lymphopoiesis during hematopoiesis
Abstract
Multiple transcription factors regulate B-cell commitment, which is coordinated with myeloid-erythroid lineage differentiation. NF-κB has long been speculated to regulate early B-cell development; however, this issue remains controversial. IκB kinase-α (IKKα) is required for splenic B-cell maturation but not for BM B-cell development. In the present study, we unexpectedly found defective BM B-cell development and increased myeloid-erythroid lineages in kinase-dead IKKα (KA/KA) knock-in mice. Markedly increased cytosolic p100, an NF-κB2-inhibitory form, and reduced nuclear NF-κB p65, RelB, p50, and p52, and IKKα were observed in KA/KA splenic and BM B cells. Several B- and myeloid-erythroid-cell regulators, including Pax5, were deregulated in KA/KA BM B cells. Using fetal liver and BM congenic transplantations and deleting IKKα from early hematopoietic cells in mice, this defect was identified as being B cell-intrinsic and an early event during hematopoiesis. Reintroducing IKKα, Pax5, or combined NF-κB molecules promoted B-cell development but repressed myeloid-erythroid cell differentiation in KA/KA BM B cells. The results of the present study demonstrate that IKKα regulates B-lineage commitment via combined canonical and noncanonical NF-κB transcriptional activities to target Pax5 expression during hematopoiesis.
Figures
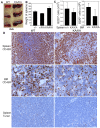
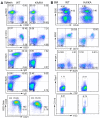

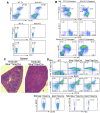



References
-
- Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. - PubMed
-
- Senftleben U, Cao Y, Xiao G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293(5534):1495–1499. - PubMed
-
- Horwitz BH, Scott ML, Cherry SR, Bronson RT, Baltimore D. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity. 1997;6(6):765–772. - PubMed
-
- Guo F, Tanzer S, Busslinger M, Weih F. Lack of nuclear factor-kappa B2/p100 causes a RelB-dependent block in early B lymphopoiesis. Blood. 2008;112(3):551–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

