Ubiquitin-specific peptidase 9, X-linked (USP9X) modulates activity of mammalian target of rapamycin (mTOR)
- PMID: 22544753
- PMCID: PMC3375539
- DOI: 10.1074/jbc.M111.328021
Ubiquitin-specific peptidase 9, X-linked (USP9X) modulates activity of mammalian target of rapamycin (mTOR)
Abstract
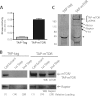
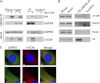
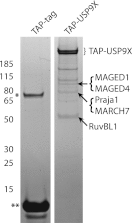
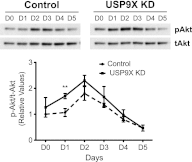
The mammalian target of rapamycin (mTOR) is an atypical serine/threonine kinase that responds to extracellular environment to regulate a number of cellular processes. These include cell growth, proliferation, and differentiation. Although both kinase-dependent and -independent functions of mTOR are known to be critical modulators of muscle cell differentiation and regeneration, the signaling mechanisms regulating mTOR activity during differentiation are still unclear. In this study we identify a novel mTOR interacting protein, the ubiquitin-specific protease USP9X, which acts as a negative regulator of mTOR activity and muscle differentiation. USP9X can co-immunoprecipitate mTOR with both Raptor and Rictor, components of mTOR complexes 1 and 2 (mTORC1 and -2), respectively, suggesting that it is present in both mTOR complexes. Knockdown of USP9X leads to increased mTORC1 activity in response to growth factor stimulation. Interestingly, upon initiation of differentiation of C2C12 mouse skeletal myoblasts, knockdown of USP9X increases mTORC2 activity. This increase in mTORC2 activity is accompanied by accelerated differentiation of myoblasts into myotubes. Taken together, our data describe the identification of the deubiquitinase USP9X as a novel mTORC1 and -2 binding partner that negatively regulates mTOR activity and skeletal muscle differentiation.
Figures



References
-
- Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 - PubMed
-
- Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 - PubMed
-
- Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin-insensitive. Nat. Cell Biol. 6, 1122–1128 - PubMed
-
- Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

