CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice
- PMID: 22544759
- PMCID: PMC3427419
- DOI: 10.1002/hep.25817
CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice
Abstract
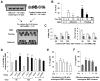
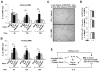
Clinical trials and animal models suggest that infusion of bone marrow cells (BMCs) is effective therapy for liver fibrosis, but the underlying mechanisms are obscure, especially those associated with early effects of BMCs. Here, we analyzed the early impact of BMC infusion and identified the subsets of BMCs showing antifibrotic effects in mice with carbon tetrachloride-induced liver fibrosis. An interaction between BMCs and activated hepatic stellate cells (HSCs) was investigated using an in vitro coculturing system. Within 24 hours, infused BMCs were in close contact with activated HSCs, which was associated with reduced liver fibrosis, enhanced hepatic expression of interleukin (IL)-10, and expanded regulatory T cells but decreased macrophage infiltration in the liver at 24 hours after BMC infusion. In contrast, IL-10-deficient (IL-10(-/-) ) BMCs failed to reproduce these effects in fibrotic livers. Intriguingly, in isolated cells, CD11b(+) Gr1(high) F4/80(-) and CD11b(+) Gr1(+) F4/80(+) BMCs expressed more IL-10 after coculturing with activated HSCs, leading to suppressed expression of collagen and α-smooth muscle actin in HSCs. Moreover, these effects were either enhanced or abrogated, respectively, when BMCs were cocultured with IL-6(-/-) and retinaldehyde dehydrogenase 1(-/-) HSCs. Similar to murine data, human BMCs expressed more IL-10 after coculturing with human HSC lines (LX-2 or hTERT), and serum IL-10 levels were significantly elevated in patients with liver cirrhosis after autologous BMC infusion.
Conclusion: Activated HSCs increase IL-10 expression in BMCs (CD11b(+) Gr1(high) F4/80(-) and CD11b(+) Gr1(+) F4/80(+) cells), which in turn ameliorates liver fibrosis. Our findings could enhance the design of BMC therapy for liver fibrosis.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures





References
-
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. - PubMed
-
- Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. - PubMed
-
- Terai S, Ishikawa T, Omori K, Aoyama K, Marumoto Y, Urata Y, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. - PubMed
-
- Biermann H, Pietz B, Dreier R, Schmid KW, Sorg C, Sunderkotter C. Murine leukocytes with ring-shaped nuclei include granulocytes, monocytes, and their precursors. J Leukoc Biol. 1999;65:217–231. - PubMed
-
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
