Lysine acetylation is widespread on proteins of diverse function and localization in the protozoan parasite Toxoplasma gondii
- PMID: 22544907
- PMCID: PMC3370464
- DOI: 10.1128/EC.00088-12
Lysine acetylation is widespread on proteins of diverse function and localization in the protozoan parasite Toxoplasma gondii
Abstract
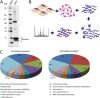
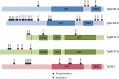
While histone proteins are the founding members of lysine acetylation substrates, it is now clear that hundreds of other proteins can be acetylated in multiple compartments of the cell. Our knowledge of the scope of this modification throughout the kingdom of life is beginning to emerge, as proteome-wide lysine acetylation has been documented in prokaryotes, Arabidopsis thaliana, Drosophila melanogaster, and human cells. Using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to identify parasite peptides enriched by immunopurification with acetyl-lysine antibody, we produced the first proteome-wide analysis of acetylation for a protozoan organism, the opportunistic apicomplexan parasite Toxoplasma gondii. The results show that lysine acetylation is abundant in the actively proliferating tachyzoite form of the parasite, which causes acute toxoplasmosis. Our approach successfully identified known acetylation marks on Toxoplasma histones and α-tubulin and detected over 400 novel acetylation sites on a wide variety of additional proteins, including those with roles in transcription, translation, metabolism, and stress responses. Importantly, an extensive set of parasite-specific proteins, including those found in organelles unique to Apicomplexa, is acetylated in the parasite. Our data provide a wealth of new information that improves our understanding of the evolution of this vital regulatory modification while potentially revealing novel therapeutic avenues. We conclude from this study that lysine acetylation was prevalent in the early stages of eukaryotic cell evolution and occurs on proteins involved in a remarkably diverse array of cellular functions, including those that are specific to parasites.
Figures

References
-
- Braun L, et al. 2009. The small ubiquitin-like modifier (SUMO)-conjugating system of Toxoplasma gondii. Int. J. Parasitol. 39:81–90 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

