Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function
- PMID: 22545799
- PMCID: PMC3421059
- DOI: 10.1111/j.1574-6976.2012.00342.x
Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function
Abstract
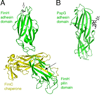
Gram-negative bacteria express a wide variety of organelles on their cell surface. These surface structures may be the end products of secretion systems, such as the hair-like fibers assembled by the chaperone/usher (CU) and type IV pilus pathways, which generally function in adhesion to surfaces and bacterial-bacterial and bacterial-host interactions. Alternatively, the surface organelles may be integral components of the secretion machinery itself, such as the needle complex and pilus extensions formed by the type III and type IV secretion systems, which function in the delivery of bacterial effectors inside host cells. Bacterial surface structures perform functions critical for pathogenesis and have evolved to withstand forces exerted by the external environment and cope with defenses mounted by the host immune system. Given their essential roles in pathogenesis and exposed nature, bacterial surface structures also make attractive targets for therapeutic intervention. This review will describe the structure and function of surface organelles assembled by four different Gram-negative bacterial secretion systems: the CU pathway, the type IV pilus pathway, and the type III and type IV secretion systems.
© 2012 Federation of European Microbiological Societies. Published by Blackwell Publishing Ltd. All rights reserved.
Figures








References
-
- Aas FE, Lovold C, Koomey M. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to Type IV pilus expression. Mol Microbiol. 2002;46:1441–1450. - PubMed
-
- Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobactericeae. Nature. 1988;336:682–684. - PubMed
-
- Akeda Y, Galan JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

