Detecting and destroying cancer cells in more than one way with noble metals and different confinement properties on the nanoscale
- PMID: 22546051
- PMCID: PMC4706153
- DOI: 10.1021/ar2003122
Detecting and destroying cancer cells in more than one way with noble metals and different confinement properties on the nanoscale
Abstract
Today, 1 in 2 males and 1 in 3 females in the United States will develop cancer at some point during their lifetimes, and 1 in 4 males and 1 in 5 females in the United States will die from the disease. New methods for detection and treatment have dramatically improved cancer care in the United States. However, as improved detection and increasing exposure to carcinogens has led to higher rates of cancer incidence, clinicians and researchers have not balanced that increase with a similar decrease in cancer mortality rates. This mismatch highlights a clear and urgent need for increasingly potent and selective methods with which to detect and treat cancers at their earliest stages. Nanotechnology, the use of materials with structural features ranging from 1 to 100 nm in size, has dramatically altered the design, use, and delivery of cancer diagnostic and therapeutic agents. The unique and newly discovered properties of these structures can enhance the specificities with which biomedical agents are delivered, complementing their efficacy or diminishing unintended side effects. Gold (and silver) nanotechnologies afford a particularly unique set of physiological and optical properties which can be leveraged in applications ranging from in vitro/vivo therapeutics and drug delivery to imaging and diagnostics, surgical guidance, and treatment monitoring. Nanoscale diagnostic and therapeutic agents have been in use since the development of micellar nanocarriers and polymer-drug nanoconjugates in the mid-1950s, liposomes by Bangham and Watkins in the mid-1960s, and the introduction of polymeric nanoparticles by Langer and Folkman in 1976. Since then, nanoscale constructs such as dendrimers, protein nanoconjugates, and inorganic nanoparticles have been developed for the systemic delivery of agents to specific disease sites. Today, more than 20 FDA-approved diagnostic or therapeutic nanotechnologies are in clinical use with roughly 250 others in clinical development. The global market for nano-enabled medical technologies is expected to grow to $70-160 billion by 2015, rivaling the current market share of biologics worldwide. In this Account, we explore the emerging applications of noble metal nanotechnologies in cancer diagnostics and therapeutics carried out by our group and by others. Many of the novel biomedical properties associated with gold and silver nanoparticles arise from confinement effects: (i) the confinement of photons within the particle which can lead to dramatic electromagnetic scattering and absorption (useful in sensing and heating applications, respectively); (ii) the confinement of molecules around the nanoparticle (useful in drug delivery); and (iii) the cellular/subcellular confinement of particles within malignant cells (such as selective, nuclear-targeted cytotoxic DNA damage by gold nanoparticles). We then describe how these confinement effects relate to specific aspects of diagnosis and treatment such as (i) laser photothermal therapy, optical scattering microscopy, and spectroscopic detection, (ii) drug targeting and delivery, and (iii) the ability of these structures to act as intrinsic therapeutic agents which can selectively perturb/inhibit cellular functions such as division. We intend to provide the reader with a unique physical and chemical perspective on both the design and application of these technologies in cancer diagnostics and therapeutics. We also suggest a framework for approaching future research in the field.
Figures


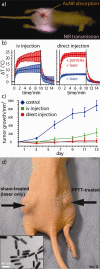

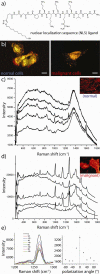
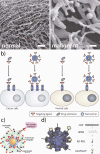
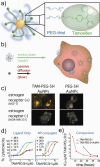
References
-
-
For example, while gold nanoparticle surfacesa can be routinely functionalized with active ligands (i.e. not stabilizers) at densities approximately 1.0 × 106 molecules/ m2, those typically attainable using more conventional liposomesb and PLGA (poly(lactic-co-glycolic acid)) nanoparticlesc are 2 to 3 orders of magnitude less, 1.2 × 104 and 4.4 × 103 molecules/ m2, respectively, allowing for augmented targeting affinity, avidity, and multifunctionality from gold nanostrucures, as well as drug loading densities comperable to those attainable using nanoencapsulation methods. Bard AJ, Faulkner LR. Electrochemical Methods: Fundamentals and Applications. 2nd ed. John Wiley & Sons, Inc.; New York: 2001. Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8786–8791.Park J, Mattessich T, Jay SM, Agawu A, Saltzman WM, Fahmy TM. Enhancement of surface ligand display on PLGA nanoparticles with amphiphilic ligand conjugates. J. Control. Release. 2011;156:109–115.
-
-
- Oyelere AK, El-Sayed MA, Dreaden EC. Targeted Cellular Delivery of Nanoparticles. 2010 U.S. patent 12/890,519 pending.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

