Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis
- PMID: 22546052
- PMCID: PMC3397122
- DOI: 10.1089/ten.TEA.2011.0127
Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis
Abstract
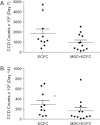
Mesenchymal stem cells (MSCs) seeded in composite implants formed of hydroxyapatite (HA) and poly (lactide-co-glycolide) (PLG) exhibit increased osteogenesis and enhanced angiogenic potential. Endothelial colony-forming cells (ECFCs) can participate in de novo vessel formation when implanted in vivo. The aim of this study was to determine the capacity of HA-PLG composites to cotransplant MSCs and ECFCs, with the goal of accelerating vascularization and resultant bone formation. The incorporation of HA into PLG scaffolds improved the efficiency of cell seeding and ECFC survival in vitro. We observed increases in mRNA expression and secretion of potent angiogenic factors by MSCs when cultured on HA-PLG scaffolds compared to PLG controls. Upon implantation into an orthotopic calvarial defect, ECFC survival on composite scaffolds was not increased in the presence of MSCs, nor did the addition of ECFCs enhance vascularization beyond increases observed with MSCs alone. Microcomputed tomography (micro-CT) performed on explanted calvarial tissues after 12 weeks revealed no significant differences between treatment groups for bone volume fraction (BVF) or bone mineral density (BMD). Taken together, these results provide evidence that HA-containing composite scaffolds seeded with MSCs can enhance neovascularization, yet MSC-secreted trophic factors do not consistently increase the persistence of co-transplanted ECFCs.
Figures





References
-
- Kaigler D. Krebsbach P.H. Wang Z. West E.R. Horger K. Mooney D.J. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633. - PubMed
-
- Peng H. Usas A. Olshanski A. Ho A.M. Gearhart B. Cooper G.M., et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

