The effect of efavirenz versus nevirapine-containing regimens on immunologic, virologic and clinical outcomes in a prospective observational study
- PMID: 22546987
- PMCID: PMC3647467
- DOI: 10.1097/QAD.0b013e328354f497
The effect of efavirenz versus nevirapine-containing regimens on immunologic, virologic and clinical outcomes in a prospective observational study
Abstract
Objective: To compare regimens consisting of either efavirenz or nevirapine and two or more nucleoside reverse transcriptase inhibitors (NRTIs) among HIV-infected, antiretroviral-naive, and AIDS-free individuals with respect to clinical, immunologic, and virologic outcomes.
Design: Prospective studies of HIV-infected individuals in Europe and the US included in the HIV-CAUSAL Collaboration.
Methods: Antiretroviral therapy-naive and AIDS-free individuals were followed from the time they started an NRTI, efavirenz or nevirapine, classified as following one or both types of regimens at baseline, and censored when they started an ineligible drug or at 6 months if their regimen was not yet complete. We estimated the 'intention-to-treat' effect for nevirapine versus efavirenz regimens on clinical, immunologic, and virologic outcomes. Our models included baseline covariates and adjusted for potential bias introduced by censoring via inverse probability weighting.
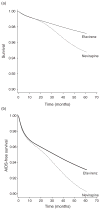
Results: A total of 15 336 individuals initiated an efavirenz regimen (274 deaths, 774 AIDS-defining illnesses) and 8129 individuals initiated a nevirapine regimen (203 deaths, 441 AIDS-defining illnesses). The intention-to-treat hazard ratios [95% confidence interval (CI)] for nevirapine versus efavirenz regimens were 1.59 (1.27, 1.98) for death and 1.28 (1.09, 1.50) for AIDS-defining illness. Individuals on nevirapine regimens experienced a smaller 12-month increase in CD4 cell count by 11.49 cells/μl and were 52% more likely to have virologic failure at 12 months as those on efavirenz regimens.
Conclusions: Our intention-to-treat estimates are consistent with a lower mortality, a lower incidence of AIDS-defining illness, a larger 12-month increase in CD4 cell count, and a smaller risk of virologic failure at 12 months for efavirenz compared with nevirapine.
© 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins
Conflict of interest statement
The research was partly supported by NIH grants R01-AI073127, U10-AA013566, and MRC grant G0700820. VACS was supported by AHRQ grant R01-HS018372 and NIH grants U10-AA13566, U24-AA020794, U01-AA020790), R01-HL095136, R01-HL090342, RCI-HL100347, U01-A1069918 and P30-MH062294, and by the Veterans Health Administration Office of Research and Development (VA REA 08-266, VA IRR Merit Award) and Office of Academic Affiliations (Medical Informatics Fellowship). Jonathan Sterne has received travel grants from GlaxoSmithKline and honoraria from Gilead Sciences. Caroline Sabin has received travel grants, fees for speaking and honoraria from various pharmaceutical companies including Bristol Myers Squibb, Gilead Sciences, Boehringer-Ingelheim, Janssen Pharmaceutica, and Tibotec. Dominique Cost-agliola has received travel grants, consultancy fees, honoraria or study grants from various pharmaceutical companies including Abbott, Boehringer-Ingelheim, Bristol-Myers-Squibb, Gilead Sciences, GlaxoSmith-Kline, Janssen, Merck and Roche. Heiner C. Bucher has received travel grants, honoraria and unrestricted research grants from GlaxoSmithKline, Bristol-Myers-Squibb, Gilead, Roche, Abbott, Tibotec, Janssen, Boehringer-Ingelheim and ViiV Healthcare. Laurence Meyer has received honoraria from GlaxoSmithKline. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Sophie Abgrall has received travel grants, consultancy fees, fees for speaking from various pharmaceutical companies including Abbott, Boehringer-Ingelheim, Bristol-Myers-Squibb, Gilead Sciences, GlaxoSmithKline and Janssen.
Figures
References
-
- World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009 http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. - PubMed
-
- European AIDS Clinical Society. EACS Guidelines. 2011 http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsg....
-
- Gazzard BG, Anderson J, Babiker A, Boffito M, Brook G, Brough G, et al. 1; British HIV Association Guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Med. 2008;9:563–608. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; 2011. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
-
- Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 304:321–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-AI073127/AI/NIAID NIH HHS/United States
- R01-HL095136/HL/NHLBI NIH HHS/United States
- P30 MH062294/MH/NIMH NIH HHS/United States
- R01 HL090342/HL/NHLBI NIH HHS/United States
- G0700820/MRC_/Medical Research Council/United Kingdom
- R01 HS018372/HS/AHRQ HHS/United States
- U10-AA013566/AA/NIAAA NIH HHS/United States
- R01-HL090342/HL/NHLBI NIH HHS/United States
- U01-AA020790/AA/NIAAA NIH HHS/United States
- U01-A1069918/PHS HHS/United States
- RCI-HL100347/HL/NHLBI NIH HHS/United States
- RC1 HL100347/HL/NHLBI NIH HHS/United States
- R01 HL095136/HL/NHLBI NIH HHS/United States
- G0900274/MRC_/Medical Research Council/United Kingdom
- U10 AA013566/AA/NIAAA NIH HHS/United States
- R01-HS018372/HS/AHRQ HHS/United States
- P30-MH062294/MH/NIMH NIH HHS/United States
- R37 AI032475/AI/NIAID NIH HHS/United States
- U01 AA020790/AA/NIAAA NIH HHS/United States
- U24-AA020794/AA/NIAAA NIH HHS/United States
- R01 AI073127/AI/NIAID NIH HHS/United States
- U24 AA020794/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

