Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity
- PMID: 22547072
- PMCID: PMC3397903
- DOI: 10.1074/jbc.M111.329763
Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity
Abstract
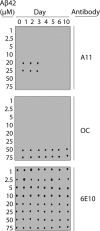
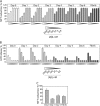
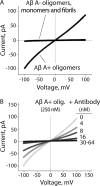
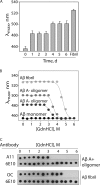
Several protein conformational disorders (Parkinson and prion diseases) are linked to aberrant folding of proteins into prefibrillar oligomers and amyloid fibrils. Although prefibrillar oligomers are more toxic than their fibrillar counterparts, it is difficult to decouple the origin of their dissimilar toxicity because oligomers and fibrils differ both in terms of structure and size. Here we report the characterization of two oligomers of the 42-residue amyloid β (Aβ42) peptide associated with Alzheimer disease that possess similar size and dissimilar toxicity. We find that Aβ42 spontaneously forms prefibrillar oligomers at Aβ concentrations below 30 μm in the absence of agitation, whereas higher Aβ concentrations lead to rapid formation of fibrils. Interestingly, Aβ prefibrillar oligomers do not convert into fibrils under quiescent assembly conditions but instead convert into a second type of oligomer with size and morphology similar to those of Aβ prefibrillar oligomers. Strikingly, this alternative Aβ oligomer is non-toxic to mammalian cells relative to Aβ monomer. We find that two hydrophobic peptide segments within Aβ (residues 16-22 and 30-42) are more solvent-exposed in the more toxic Aβ oligomer. The less toxic oligomer is devoid of β-sheet structure, insoluble, and non-immunoreactive with oligomer- and fibril-specific antibodies. Moreover, the less toxic oligomer is incapable of disrupting lipid bilayers, in contrast to its more toxic oligomeric counterpart. Our results suggest that the ability of non-fibrillar Aβ oligomers to interact with and disrupt cellular membranes is linked to the degree of solvent exposure of their central and C-terminal hydrophobic peptide segments.
Figures


References
-
- Kirkitadze M. D., Bitan G., Teplow D. B. (2002) Paradigm shifts in Alzheimer's disease and other neurodegenerative disorders. The emerging role of oligomeric assemblies. J. Neurosci. Res. 69, 567–577 - PubMed
-
- Sakono M., Zako T. (2010) Amyloid oligomers. Formation and toxicity of Aβ oligomers. FEBS J. 277, 1348–1358 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

