Synthesis and biological evaluation of new ligustrazine derivatives as anti-tumor agents
- PMID: 22547319
- PMCID: PMC6268357
- DOI: 10.3390/molecules17054972
Synthesis and biological evaluation of new ligustrazine derivatives as anti-tumor agents
Abstract
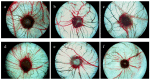
To discover new anti-cancer agents with multi-effect and low toxicity, a series of ligustrazine derivatives were synthesized using several effective anti-tumor ingredients of Shiquandabu Wan as starting materials. Our idea was enlightened by the "combination principle" in drug discovery. The ligustrazine derivatives' anti-tumor activities were evaluated on the HCT-8, Bel-7402, BGC-823, A-549 and A2780 human cancer cell lines. In addition the angiogenesis activities were valued by the chick chorioallantoic membrane (CAM) assay. 1,7-bis(4-(3,5,6-Trimethylpyrazin-2-yl)-3-methoxyphenyl)-1,6-heptadiene-3,5-dione (4) and 3 α,12 α-dihydroxy-5β-dholanic acid-3,5,6-trimethylpyrazin-2-methyl ester (5) not only displayed antiproliferative activities on these cancer cells, but also dramatically suppressed normal angiogenesis in CAM. The LD₅₀ value of the compound 5 exceeded 3.0 g/kg by oral administration in mice.
Figures
Similar articles
-
Synthesis and evaluation of 2,4-disubstituted quinazoline derivatives with potent anti-angiogenesis activities.Molecules. 2014 Jun 26;19(7):8916-32. doi: 10.3390/molecules19078916. Molecules. 2014. PMID: 24972275 Free PMC article.
-
Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells.Molecules. 2018 Jan 5;23(1):110. doi: 10.3390/molecules23010110. Molecules. 2018. PMID: 29303970 Free PMC article.
-
Solid phase synthesis and biological evaluation of probestin as an angiogenesis inhibitor.Bioorg Med Chem Lett. 2013 Jun 15;23(12):3561-4. doi: 10.1016/j.bmcl.2013.04.031. Epub 2013 Apr 23. Bioorg Med Chem Lett. 2013. PMID: 23664876 Free PMC article.
-
The chick embryo chorioallantoic membrane (CAM) assay.Reprod Toxicol. 2017 Jun;70:97-101. doi: 10.1016/j.reprotox.2016.11.004. Epub 2016 Nov 8. Reprod Toxicol. 2017. PMID: 27832950 Review.
-
The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents.Curr Cancer Drug Targets. 2005 Jun;5(4):249-66. doi: 10.2174/1568009054064624. Curr Cancer Drug Targets. 2005. PMID: 15975046 Review.
Cited by
-
Natural Products-Pyrazine Hybrids: A Review of Developments in Medicinal Chemistry.Molecules. 2023 Nov 5;28(21):7440. doi: 10.3390/molecules28217440. Molecules. 2023. PMID: 37959859 Free PMC article. Review.
-
Tetramethylpyrazine: A Review of Its Antitumor Potential and Mechanisms.Front Pharmacol. 2021 Dec 16;12:764331. doi: 10.3389/fphar.2021.764331. eCollection 2021. Front Pharmacol. 2021. PMID: 34975475 Free PMC article. Review.
-
Sichong formula inhibits the proliferation and migration of human gastric cancer cells.Onco Targets Ther. 2019 Jul 16;12:5741-5750. doi: 10.2147/OTT.S199605. eCollection 2019. Onco Targets Ther. 2019. PMID: 31410020 Free PMC article.
-
[A Novel Chinese Medicine Formula Inhibits Non-small Cell Lung Cancer by Triggering Oxidative Stress Dependent on Pentose Phosphate Pathway].Zhongguo Fei Ai Za Zhi. 2023 Sep 20;26(9):639-649. doi: 10.3779/j.issn.1009-3419.2023.101.27. Zhongguo Fei Ai Za Zhi. 2023. PMID: 37985150 Free PMC article. Chinese.
-
In Vitro and In Vivo Inhibitory Effect of Gujin Xiaoliu Tang in Non-Small Cell Lung Cancer.Evid Based Complement Alternat Med. 2018 Sep 9;2018:8936108. doi: 10.1155/2018/8936108. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30271456 Free PMC article.
References
-
- Fialho A.M., Gupta T.K.D., Chakrabarty A.M. Designing promiscuous drugs? Look at what nature made. Lett. Drug Des. Discov. 2007;4:40–43. doi: 10.2174/157018007778992946. - DOI
-
- Wu D.H., Xu X.J. A new practice: Study on the molecular mechanism of traditional Chinese medicine by computational pharmacology methods: Part 1: Pharmacokinetic modeling and chemical space distribution. Lett. Drug Des. Discov. 2011;8:652–658. doi: 10.2174/157018011796235266. - DOI
-
- Wu D.H., Xu X.J., Zhang M.Z., Wang L. A new practice: Study on the molecular mechanism of traditional Chinese medicine by computational pharmacology methods: Part 2: Pharmacodynamic modeling and distribution on ligand-target space of effective components. Lett. Drug Des. Discov. 2011;8:1009–1014. doi: 10.2174/157018011797655331. - DOI
-
- Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L., Dong J.P., Liang L.R., Li X.W., Hu K., et al. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: A randomized trial. Ann. Intern. Med. 2011;155:217–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

