Matrix nanotopography as a regulator of cell function
- PMID: 22547406
- PMCID: PMC3341161
- DOI: 10.1083/jcb.201108062
Matrix nanotopography as a regulator of cell function
Abstract
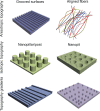
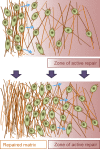
The architecture of the extracellular matrix (ECM) directs cell behavior by providing spatial and mechanical cues to which cells respond. In addition to soluble chemical factors, physical interactions between the cell and ECM regulate primary cell processes, including differentiation, migration, and proliferation. Advances in microtechnology and, more recently, nanotechnology provide a powerful means to study the influence of the ECM on cell behavior. By recapitulating local architectures that cells encounter in vivo, we can elucidate and dissect the fundamental signal transduction pathways that control cell behavior in critical developmental, physiological, and pathological processes.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

