Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale
- PMID: 22547603
- PMCID: PMC3675696
- DOI: 10.1200/JCO.2011.39.1110
Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale
Abstract
Purpose: The platinum chemotherapy agents cisplatin and carboplatin are widely used in the treatment of adult and pediatric cancers. Cisplatin causes hearing loss in at least 60% of pediatric patients. Reducing cisplatin and high-dose carboplatin ototoxicity without reducing efficacy is important.
Patients and methods: This review summarizes recommendations made at the 42nd Congress of the International Society of Pediatric Oncology (SIOP) in Boston, October 21-24, 2010, reflecting input from international basic scientists, pediatric oncologists, otolaryngologists, oncology nurses, audiologists, and neurosurgeons to develop and advance research and clinical trials for otoprotection.
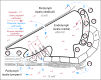
Results: Platinum initially impairs hearing in the high frequencies and progresses to lower frequencies with increasing cumulative dose. Genes involved in drug transport, metabolism, and DNA repair regulate platinum toxicities. Otoprotection can be achieved by acting on several these pathways and generally involves antioxidant thiol agents. Otoprotection is a strategy being explored to decrease hearing loss while maintaining dose intensity or allowing dose escalation, but it has the potential to interfere with tumoricidal effects. Route of administration and optimal timing relative to platinum therapy are critical issues. In addition, international standards for grading and comparing ototoxicity are essential to the success of prospective pediatric trials aimed at reducing platinum-induced hearing loss.
Conclusion: Collaborative prospective basic and clinical trial research is needed to reduce the incidence of irreversible platinum-induced hearing loss, and optimize cancer control. Wide use of the new internationally agreed-on SIOP Boston ototoxicity scale in current and future otoprotection trials should help facilitate this goal.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
New International Society of Pediatric Oncology Boston Ototoxicity Grading Scale for pediatric oncology: still room for improvement.J Clin Oncol. 2012 Jul 1;30(19):2303-6. doi: 10.1200/JCO.2011.41.3187. Epub 2012 Apr 30. J Clin Oncol. 2012. PMID: 22547588 No abstract available.
References
-
- National Cancer Institute. Cancer Drug Information: Cisplatin, May 10, 2011 update. http://www.cancer.gov/cancertopics/druginfo/cisplatin.
-
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. - PubMed
-
- Knight KR, Kraemer DF, Winter C, et al. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. - PubMed
-
- Doolittle ND, Muldoon LL, Brummett RE, et al. Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin Cancer Res. 2001;7:493–500. - PubMed
-
- Neuwelt EA, Brummett RE, Doolittle ND, et al. First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J Pharmacol Exp Ther. 1998;286:77–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

