Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation
- PMID: 22547651
- PMCID: PMC3348099
- DOI: 10.1084/jem.20112583
Resident CD141 (BDCA3)+ dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation
Abstract
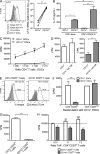
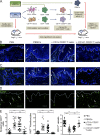
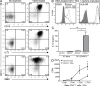
Human skin immune homeostasis, and its regulation by specialized subsets of tissue-residing immune sentinels, is poorly understood. In this study, we identify an immunoregulatory tissue-resident dendritic cell (DC) in the dermis of human skin that is characterized by surface expression of CD141, CD14, and constitutive IL-10 secretion (CD141(+) DDCs). CD141(+) DDCs possess lymph node migratory capacity, induce T cell hyporesponsiveness, cross-present self-antigens to autoreactive T cells, and induce potent regulatory T cells that inhibit skin inflammation. Vitamin D(3) (VitD3) promotes certain phenotypic and functional properties of tissue-resident CD141(+) DDCs from human blood DCs. These CD141(+) DDC-like cells can be generated in vitro and, once transferred in vivo, have the capacity to inhibit xeno-graft versus host disease and tumor alloimmunity. These findings suggest that CD141(+) DDCs play an essential role in the maintenance of skin homeostasis and in the regulation of both systemic and tumor alloimmunity. Finally, VitD3-induced CD141(+) DDC-like cells have potential clinical use for their capacity to induce immune tolerance.
Figures


References
-
- Azukizawa H., Döhler A., Kanazawa N., Nayak A., Lipp M., Malissen B., Autenrieth I., Katayama I., Riemann M., Weih F., et al. 2011. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells. Eur. J. Immunol. 41:1420–1434 10.1002/eji.201040930 - DOI - PubMed
-
- Bachem A., Güttler S., Hartung E., Ebstein F., Schaefer M., Tannert A., Salama A., Movassaghi K., Opitz C., Mages H.W., et al. 2010. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 207:1273–1281 10.1084/jem.20100348 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

