B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells
- PMID: 22547654
- PMCID: PMC3348102
- DOI: 10.1084/jem.20111675
B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells
Abstract
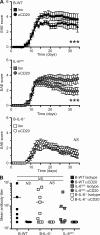
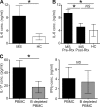
B cells have paradoxical roles in autoimmunity, exerting both pathogenic and protective effects. Pathogenesis may be antibody independent, as B cell depletion therapy (BCDT) leads to amelioration of disease irrespective of autoantibody ablation. However, the mechanisms of pathogenesis are poorly understood. We demonstrate that BCDT alleviates central nervous system autoimmunity through ablation of IL-6-secreting pathogenic B cells. B cells from mice with experimental autoimmune encephalomyelitis (EAE) secreted elevated levels of IL-6 compared with B cells from naive controls, and mice with a B cell-specific IL-6 deficiency showed less severe disease than mice with wild-type B cells. Moreover, BCDT ameliorated EAE only in mice with IL-6-sufficient B cells. This mechanism of pathogenesis may also operate in multiple sclerosis (MS) because B cells from MS patients produced more IL-6 than B cells from healthy controls, and this abnormality was normalized with B cell reconstitution after Rituximab treatment. This suggests that BCDT improved disease progression, at least partly, by eliminating IL-6-producing B cells in MS patients. Taking these data together, we conclude that IL-6 secretion is a major mechanism of B cell-driven pathogenesis in T cell-mediated autoimmune disease such as EAE and MS.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

