Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse
- PMID: 22547824
- PMCID: PMC3356680
- DOI: 10.1073/pnas.1205869109
Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse
Abstract
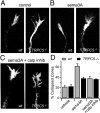
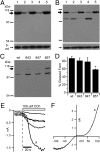
The nonselective cation channel transient receptor potential canonical (TRPC)5 is found predominantly in the brain and has been proposed to regulate neuronal processes and growth cones. Here, we demonstrate that semaphorin 3A-mediated growth cone collapse is reduced in hippocampal neurons from TRPC5 null mice. This reduction is reproduced by inhibition of the calcium-sensitive protease calpain in wild-type neurons but not in TRPC5(-/-) neurons. We show that calpain-1 and calpain-2 cleave and functionally activate TRPC5. Mutation of a critical threonine at position 857 inhibits calpain-2 cleavage of the channel. Finally, we show that the truncated TRPC5 predicted to result from calpain cleavage is functionally active. These results indicate that semaphorin 3A initiates growth cone collapse via activation of calpain that in turn potentiates TRPC5 activity. Thus, TRPC5 acts downstream of semaphorin signaling to cause changes in neuronal growth cone morphology and nervous system development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. - PubMed
-
- Sakai T, et al. Mouse semaphorin H induces PC12 cell neurite outgrowth activating Ras-mitogen-activated protein kinase signaling pathway via Ca2+ influx. J Biol Chem. 1999;274:29666–29671. - PubMed
-
- To KC, Church J, O’Connor TP. Combined activation of calpain and calcineurin during ligand-induced growth cone collapse. Mol Cell Neurosci. 2007;36:425–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

