Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown
- PMID: 22548113
- PMCID: PMC3324142
- DOI: 10.1155/2012/187585
Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown
Abstract
The best hope of controlling the herpes simplex virus type 1 and type 2 (HSV-1 and HSV-2) pandemic is the development of an effective vaccine. However, in spite of several clinical trials, starting as early as 1920s, no vaccine has been proven sufficiently safe and efficient to warrant commercial development. In recent years, great strides in cellular and molecular immunology have stimulated creative efforts in controlling herpes infection and disease. However, before moving towards new vaccine strategy, it is necessary to answer two fundamental questions: (i) why past herpes vaccines have failed? (ii) Why the majority of HSV seropositive individuals (i.e., asymptomatic individuals) are naturally "protected" exhibiting few or no recurrent clinical disease, while other HSV seropositive individuals (i.e., symptomatic individuals) have frequent ocular, orofacial, and/or genital herpes clinical episodes? We recently discovered several discrete sets of HSV-1 symptomatic and asymptomatic epitopes recognized by CD4(+) and CD8(+) T cells from seropositive symptomatic versus asymptomatic individuals. These asymptomatic epitopes will provide a solid foundation for the development of novel herpes epitope-based vaccine strategy. Here we provide a brief overview of past clinical vaccine trials, outline current progress towards developing a new generation "asymptomatic" clinical herpes vaccines, and discuss future mucosal "asymptomatic" prime-boost vaccines that could optimize local protective immunity.
Figures
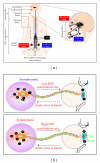

References
-
- Rubbo PA, Tuaillon E, Nagot N, et al. HIV-1 infection impairs HSV-specific CD4+ and CD8+ T-cell response by reducing Th1 cytokines and CCR5 ligand secretion. Journal of Acquired Immune Deficiency Syndromes. 2011;58(1):9–17. - PubMed
-
- Dasgupta G, Chentoufi AA, You S, et al. Engagement of TLR2 reverses the suppressor function of conjunctiva CD4+CD25+ regulatory T cells and promotes herpes simplex virus epitope-specific CD4+CD25- effector T cell responses. Investigative Ophthalmology & Visual Science. 2011;52(6):3321–3333. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

