Influenza serological studies to inform public health action: best practices to optimise timing, quality and reporting
- PMID: 22548725
- PMCID: PMC5855149
- DOI: 10.1111/j.1750-2659.2012.0370a.x
Influenza serological studies to inform public health action: best practices to optimise timing, quality and reporting
Abstract
Background: Serological studies can detect infection with a novel influenza virus in the absence of symptoms or positive virology, providing useful information on infection that goes beyond the estimates from epidemiological, clinical and virological data. During the 2009 A(H1N1) pandemic, an impressive number of detailed serological studies were performed, yet the majority of serological data were available only after the first wave of infection. This limited the ability to estimate the transmissibility and severity of this novel infection, and the variability in methodology and reporting limited the ability to compare and combine the serological data.
Objectives: To identify best practices for conduct and standardisation of serological studies on outbreak and pandemic influenza to inform public policy.
Methods/setting: An international meeting was held in February 2011 in Ottawa, Canada, to foster the consensus for greater standardisation of influenza serological studies.
Results: Best practices for serological investigations of influenza epidemiology include the following: classification of studies as pre-pandemic, outbreak, pandemic or inter-pandemic with a clearly identified objective; use of international serum standards for laboratory assays; cohort and cross-sectional study designs with common standards for data collection; use of serum banks to improve sampling capacity; and potential for linkage of serological, clinical and epidemiological data. Advance planning for outbreak studies would enable a rapid and coordinated response; inclusion of serological studies in pandemic plans should be considered.
Conclusions: Optimising the quality, comparability and combinability of influenza serological studies will provide important data upon emergence of a novel or variant influenza virus to inform public health action.
© 2012 Blackwell Publishing Ltd.
Figures
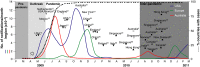
 ). The working classification for serological studies according to time of sample collection and study objective is shown in this context, by shaded regions (pre‐pandemic, outbreak, pandemic, inter‐pandemic). Location and reference (superscript) of studies over time are shown. The earliest published serological studies in 2009 described assay sensitivity and specificity (♦), the cross‐reactive pre‐pandemic antibody responses () and demonstrated a lack of protection from seasonal vaccines (*). In 2010, serological studies describing the first wave of infection (
). The working classification for serological studies according to time of sample collection and study objective is shown in this context, by shaded regions (pre‐pandemic, outbreak, pandemic, inter‐pandemic). Location and reference (superscript) of studies over time are shown. The earliest published serological studies in 2009 described assay sensitivity and specificity (♦), the cross‐reactive pre‐pandemic antibody responses () and demonstrated a lack of protection from seasonal vaccines (*). In 2010, serological studies describing the first wave of infection ( ), clinical identifiers of infection (□), household transmission (
), clinical identifiers of infection (□), household transmission ( ), risk factors for infection (•) and mitigation strategies (○) were published (shown are known serological studies until October 2010). The earliest published epidemiological outbreak studies (⋄) and animal infection and transmission studies (¥) are shown for comparison.
), risk factors for infection (•) and mitigation strategies (○) were published (shown are known serological studies until October 2010). The earliest published epidemiological outbreak studies (⋄) and animal infection and transmission studies (¥) are shown for comparison.References
-
- Miller E, Hoschler K, Hardelid P et al. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross‐sectional serological study. Lancet 2010; 375:1100–1108. - PubMed
-
- Iwasenko JM, Cretikos M, Paterson DL et al. Enhanced diagnosis of pandemic (H1N1) 2009 influenza infection using molecular and serological testing in intensive care unit patients with suspected influenza. Clin Infect Dis 2010; 51:70–72. - PubMed
-
- Papenburg J, Baz M, Hamelin ME et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory‐confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis 2010; 51:1033–1041. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

