Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole-controlled randomized study (J-FOCUS)
- PMID: 22548767
- PMCID: PMC3419613
- DOI: 10.1186/1471-230X-12-42
Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole-controlled randomized study (J-FOCUS)
Abstract
Background: In Japan, treatment guidelines are lacking for patients with upper gastrointestinal symptoms. We aimed to compare the efficacy of different drugs for the treatment of uninvestigated upper gastrointestinal symptoms.
Methods: This was a randomized, open-label, parallel-group multicenter study. Helicobacter pylori-negative, endoscopically uninvestigated patients ≥ 20 years of age with upper gastrointestinal symptoms of at least moderate severity (Global Overall Symptom score [GOS] ≥ 4 on a 7-point Likert scale) were randomized to treatment with omeprazole (10 mg once daily), famotidine (10 mg twice daily), mosapride (5 mg three times daily) or teprenone (50 mg three times daily). The primary endpoint was sufficient relief of upper gastrointestinal symptoms after 4 weeks of treatment (GOS ≤ 2). UMIN clinical trial registration number: UMIN000005399.
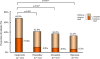
Results: Of 471 randomized patients, 454 were included in the full analysis set. After 4 weeks of treatment, sufficient symptom relief was achieved by 66.9% of patients in the omeprazole group, compared with 41.0%, 36.3% and 32.3% in the famotidine, mosapride and teprenone groups, respectively (all, p < 0.001 vs omeprazole). There were no treatment-related adverse events.
Conclusions: The favorable efficacy and safety profiles of omeprazole in relieving uninvestigated upper gastrointestinal symptoms support its use as first-line treatment in this patient group in Japan. Patients who show no improvement in symptoms despite PPI use, and those with alarm symptoms (such as vomiting, GI bleeding or acute weight loss) should receive further investigation, including prompt referral for endoscopy.
Trial registration: UMIN000005399.
Figures


References
-
- Stanghellini V. Three-month prevalence rates of gastrointestinal symptoms and the influence of demographic factors: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST) Scand J Gastroenterol Suppl. 1999;231:20–28. - PubMed
-
- Enck P, Dubois D, Marquis P. Quality of life in patients with upper gastrointestinal symptoms: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST) Scand J Gastroenterol Suppl. 1999;231:48–54. - PubMed
-
- Fujiwara Y, Higuchi K, Arafa UA, Uchida T, Tominaga K, Watanabe T, Arakawa T. Long-term effect of Helicobacter pylori eradication on quality of life, body mass index, and newly developed diseases in Japanese patients with peptic ulcer disease. Hepatogastroenterology. 2002;49:1298–1302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

