Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence
- PMID: 22549121
- PMCID: PMC3398720
- DOI: 10.1038/npp.2012.67
Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence
Abstract
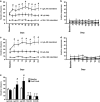
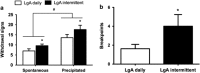
Although established smokers have a very regular pattern of smoking behavior, converging lines of evidence suggest that the escalation of smoking behavior is a critical factor in the development of dependence. However, the neurobiological mechanisms that underlie the escalation of smoking are unknown, because there is no animal model of the escalation of nicotine intake. On the basis of the pattern of smoking behavior in humans and presence of monoamine oxidase inhibitors in tobacco smoke, we hypothesized that the escalation of nicotine intake may only occur when animals are given extended-access (21 h per day) self-administration sessions after repeated periods of abstinence (24-48 h), and after chronic inhibition of monoamine oxidase using phenelzine sulfate. Intermittent access (every 24-48 h) to extended nicotine self-administration produced a robust escalation of nicotine intake, associated with increased responding under fixed- and progressive-ratio schedules of reinforcement, and increased somatic signs of withdrawal. The escalation of nicotine intake was not observed in rats with intermittent access to limited (1 h per day) nicotine self-administration or daily access to extended (21 h per day) nicotine self-administration. Moreover, inhibition of monoamine oxidase with daily administration of phenelzine increased nicotine intake by ≈ 50%. These results demonstrate that the escalation of nicotine intake only occurs in animals given intermittent periods of abstinence with extended access to nicotine, and that inhibition of monoamine oxidase may contribute to the escalation of smoking, thus validating both an animal model of the escalation of smoking behavior and the contribution of monoamine oxidase inhibition to compulsive nicotine-seeking.
Figures


References
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
-
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. - PubMed
-
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. - PubMed
-
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. - PubMed
-
- Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, Marcus MD. The return to smoking: 1-year relapse trajectories among female smokers. Nicotine Tob Res. 2005;7:533–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

