Novel data-mining methodologies for adverse drug event discovery and analysis
- PMID: 22549283
- PMCID: PMC3675775
- DOI: 10.1038/clpt.2012.50
Novel data-mining methodologies for adverse drug event discovery and analysis
Abstract
An important goal of the health system is to identify new adverse drug events (ADEs) in the postapproval period. Datamining methods that can transform data into meaningful knowledge to inform patient safety have proven essential for this purpose. New opportunities have emerged to harness data sources that have not been used within the traditional framework. This article provides an overview of recent methodological innovations and data sources used to support ADE discovery and analysis.
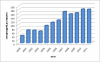
Figures


References
-
- World Health Organization . The Importance of Pharmacovigilance - Safety Monitoring of Medicinal Products. World Health Organization; Geneva: 2002.
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998 Apr 15;279(15):1200–5. - PubMed
-
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997 Jan 22;277(4):301–6. - PubMed
-
- Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009 Jun;18(6):427–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

