Noninvasive molecular imaging of neuroinflammation
- PMID: 22549622
- PMCID: PMC3390799
- DOI: 10.1038/jcbfm.2012.53
Noninvasive molecular imaging of neuroinflammation
Abstract
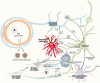
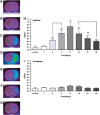
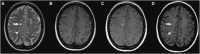
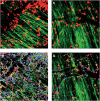
Inflammation is a highly dynamic and complex adaptive process to preserve and restore tissue homeostasis. Originally viewed as an immune-privileged organ, the central nervous system (CNS) is now recognized to have a constant interplay with the innate and the adaptive immune systems, where resident microglia and infiltrating immune cells from the periphery have important roles. Common diseases of the CNS, such as stroke, multiple sclerosis (MS), and neurodegeneration, elicit a neuroinflammatory response with the goal to limit the extent of the disease and to support repair and regeneration. However, various disease mechanisms lead to neuroinflammation (NI) contributing to the disease process itself. Molecular imaging is the method of choice to try to decipher key aspects of the dynamic interplay of various inducers, sensors, transducers, and effectors of the orchestrated inflammatory response in vivo in animal models and patients. Here, we review the basic principles of NI with emphasis on microglia and common neurologic disease mechanisms, the molecular targets which are being used and explored for imaging, and molecular imaging of NI in frequent neurologic diseases, such as stroke, MS, neurodegeneration, epilepsy, encephalitis, and gliomas.
Figures

References
-
- Abourbeh G, Thézé B, Maroy R, Dubois A, Dollé F, Tavitian B, Boisgard R.2012Imaging microglial/macrophage activation in spinal cords of experimental autoimmune encephalomyelitis (EAE) rats by PET using the mitochondrial 18-kda translocator protein (TSPO) radioligand, [18F]-DPA-714 J Neurosci(in press) - PMC - PubMed
-
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. - PubMed
-
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

