Increased resting intracellular calcium modulates NF-κB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes
- PMID: 22549782
- PMCID: PMC3375511
- DOI: 10.1074/jbc.M112.344929
Increased resting intracellular calcium modulates NF-κB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes
Abstract
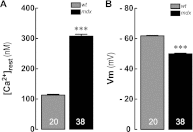
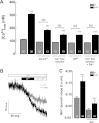
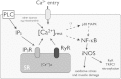
Duchenne muscular dystrophy (DMD) is a genetic disorder caused by dystrophin mutations, characterized by chronic inflammation and severe muscle wasting. Dystrophic muscles exhibit activated immune cell infiltrates, up-regulated inflammatory gene expression, and increased NF-κB activity, but the contribution of the skeletal muscle cell to this process has been unclear. The aim of this work was to study the pathways that contribute to the increased resting calcium ([Ca(2+)](rest)) observed in mdx myotubes and its possible link with up-regulation of NF-κB and pro-inflammatory gene expression in dystrophic muscle cells. [Ca(2+)](rest) was higher in mdx than in WT myotubes (308 ± 6 versus 113 ± 2 nm, p < 0.001). In mdx myotubes, both the inhibition of Ca(2+) entry (low Ca(2+) solution, Ca(2+)-free solution, and Gd(3+)) and blockade of either ryanodine receptors or inositol 1,4,5-trisphosphate receptors reduced [Ca(2+)](rest). Basal activity of NF-κB was significantly up-regulated in mdx versus WT myotubes. There was an increased transcriptional activity and p65 nuclear localization, which could be reversed when [Ca(2+)](rest) was reduced. Levels of mRNA for TNFα, IL-1β, and IL-6 were similar in WT and mdx myotubes, whereas inducible nitric-oxide synthase (iNOS) expression was increased 5-fold. Reducing [Ca(2+)](rest) using different strategies reduced iNOS gene expression presumably as a result of decreased activation of NF-κB. We propose that NF-κB, modulated by increased [Ca(2+)](rest), is constitutively active in mdx myotubes, and this mechanism can account for iNOS overexpression and the increase in reactive nitrogen species that promote damage in dystrophic skeletal muscle cells.
Figures




References
-
- Blake D. J., Weir A., Newey S. E., Davies K. E. (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 82, 291–329 - PubMed
-
- Emery A. E. (2002) The muscular dystrophies. Lancet 359, 687–695 - PubMed
-
- Henry M. D., Campbell K. P. (1996) Dystroglycan. An extracellular matrix receptor linked to the cytoskeleton. Curr. Opin. Cell Biol. 8, 625–631 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

