The bacterial outer membrane β-barrel assembly machinery
- PMID: 22549918
- PMCID: PMC3403412
- DOI: 10.1002/pro.2069
The bacterial outer membrane β-barrel assembly machinery
Abstract
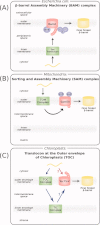
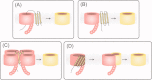
β-Barrel proteins found in the outer membrane of Gram-negative bacteria serve a variety of cellular functions. Proper folding and assembly of these proteins are essential for the viability of bacteria and can also play an important role in virulence. The β-barrel assembly machinery (BAM) complex, which is responsible for the proper assembly of β-barrels into the outer membrane of Gram-negative bacteria, has been the focus of many recent studies. This review summarizes the significant progress that has been made toward understanding the structure and function of the bacterial BAM complex.
Copyright © 2012 The Protein Society.
Figures







References
-
- Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. - PubMed
-
- Werner J, Misra R. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol Microbiol. 2005;57:1450–1459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

