Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk
- PMID: 22550134
- PMCID: PMC3413218
- DOI: 10.1194/jlr.R024075
Cholesteryl ester transfer protein inhibition as a strategy to reduce cardiovascular risk
Abstract
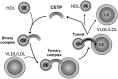
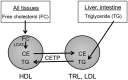
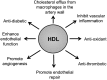
Human and rabbit plasma contain a cholesteryl ester transfer protein (CETP) that promotes net mass transfers of cholesteryl esters from high density lipoproteins (HDL) to other plasma lipoprotein fractions. As predicted, inhibition of CETP in both humans and rabbits increases the concentration of cholesterol in the potentially protective HDL fraction, while decreasing it in potentially proatherogenic non-HDL fractions. Inhibition of CETP in rabbits also inhibits the development of diet-induced atherosclerosis. However, use of the CETP inhibitor torcetrapib in humans did not reduce atheroma in three imaging trials and caused an excess of deaths and cardiovascular events in a large clinical outcome trial. The precise explanation for the harm caused by torcetrapib is unknown but may relate to documented, potentially harmful effects unrelated to inhibition of CETP. More recently, a trial using the weak CETP inhibitor dalcetrapib, which raises HDL levels less effectively than torcetrapib and does not lower non-HDL lipoprotein levels, was terminated early for reasons of futility. There was no evidence that dalcetrapib caused harm in that trial. Despite these setbacks, the hypothesis that CETP inhibitors will be antiatherogenic in humans is still being tested in studies with anacetrapib and evacetrapib, two CETP inhibitors that are much more potent than dalcetrapib and that do not share the off-target adverse effects of torcetrapib.
Figures

References
-
- Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. 2010. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681. - PMC - PubMed
-
- Barter P., Gotto A. M., LaRosa J. C., Maroni J., Szarek M., Grundy S. M., Kastelein J. J., Bittner V., Fruchart J. C. 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357: 1301–1310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

