MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome
- PMID: 22550139
- PMCID: PMC4162309
- DOI: 10.1161/CIRCRESAHA.112.268268
MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome
Abstract
Rationale: Aneurysm and dissection of the ascending thoracic aorta are the main cardiovascular complications of Marfan syndrome (MFS) resulting in premature death. Studies using mouse models of MFS have shown that activation of transforming growth factor-beta (TGF-β) and the concomitant upregulation of matrix metalloproteinases (MMPs) contribute to aneurysm development. Our previous study showed that doxycycline delayed aneurysm rupture in a mouse model of MFS, Fbn1(mgR/mgR). Losartan has been shown to prevent aneurysms in another mouse model of MFS, Fbn1(C1039G/+), through inhibition of the Erk1/2 pathway. However, the role of MMP-2 in MFS and effect of losartan on the lifespan of MFS mice remain unknown.
Objective: We investigated the role of MMP-2 in MFS and compared the effects of losartan and doxycycline on aortic dilatation and survival in Fbn1(mgR/mgR) mice.
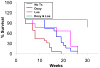
Methods and results: By life table analysis, we found that losartan and doxycycline improved the survival of Fbn1(mgR/mgR) mice. Gelatin zymography and Western blot data showed that only doxycycline inhibited MMP-2 expression, whereas both drugs decreased Erk1/2 phosphorylation. When combined, only one of nine mice died within the 30-week study; aortic histology and diameter were normalized and the effects on Smad2 phosphorylation was additive. To further explore the role of MMP-2 in MFS, we created MMP-2-deficient Fbn1(mgR/mgR) mice. MMP-2 deletion inhibited activation of TGF-β and phosphorylation of Erk1/2 and Smad2 and prolonged the lifespan of the mice.
Conclusions: These studies demonstrated that inhibition of MMP-2 by doxycycline delayed the manifestations of MFS, in part, through its ability to decrease active TGF-β and the noncanonical signaling cascade downstream of TGF-β. This study further suggested that targeting TGF-β signaling at different points might be a more effective strategy for inhibiting disease progression.
Figures




References
-
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. - PubMed
-
- Dietz HC, Pyeritz RE. Mutations in the human gene for fibrillin-1 (fbn1) in the marfan syndrome and related disorders. Hum Mol Genet. 1995;4(Spec No):1799–1809. - PubMed
-
- Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA. Life expectancy and causes of death in the marfan syndrome. N Engl J Med. 1972;286:804–808. - PubMed
-
- Ramirez F, Dietz HC. Marfan syndrome: From molecular pathogenesis to clinical treatment. Curr Opin Genet Dev. 2007 - PubMed
-
- Prokop EK, Palmer RF, Wheat MW., Jr Hydrodynamic forces in dissecting aneurysms. In-vitro studies in a tygon model and in dog aortas. Circulation research. 1970;27:121–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

