Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses
- PMID: 22550181
- PMCID: PMC3356630
- DOI: 10.1073/pnas.1118122109
Regulatory effects of mTORC2 complexes in type I IFN signaling and in the generation of IFN responses
Abstract
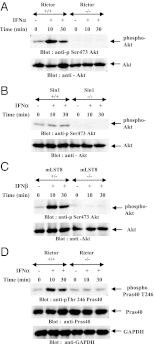
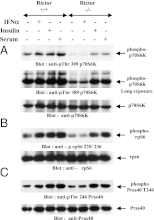
IFNs transduce signals by binding to cell surface receptors and activating cellular pathways and regulatory networks that control transcription of IFN-stimulated genes (ISGs) and mRNA translation, leading to generation of protein products that mediate biological responses. Previous studies have shown that type I IFN receptor-engaged pathways downstream of AKT and mammalian target of rapamycin complex (mTORC) 1 play important roles in mRNA translation of ISGs and the generation of IFN responses, but the roles of mTORC2 complexes in IFN signaling are unknown. We provide evidence that mTORC2 complexes control IFN-induced phosphorylation of AKT on serine 473 and their function is ultimately required for IFN-dependent gene transcription via interferon-stimulated response elements. We also demonstrate that such complexes exhibit regulatory effects on other IFN-dependent mammalian target of rapamycin-mediated signaling events, likely via engagement of the AKT/mTORC1 axis, including IFN-induced phosphorylation of S6 kinase and its effector rpS6, as well as phosphorylation of the translational repressor 4E-binding protein 1. We also show that induction of ISG protein expression and the generation of antiviral responses are defective in Rictor and mLST8-KO cells. Together, our data provide evidence for unique functions of mTORC2 complexes in the induction of type I IFN responses and suggest a critical role for mTORC2-mediated signals in IFN signaling.
Conflict of interest statement
Conflict of interest statement: D.P.B. is an employee of Biogen Idec and an owner of Biogen Idec stock.
Figures

References
-
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. - PubMed
-
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. - PubMed
-
- Parmar S, Platanias LC. Interferons: Mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15:431–439. - PubMed
-
- Javed A, Reder AT. Therapeutic role of beta-interferons in multiple sclerosis. Pharmacol Ther. 2006;110:35–56. - PubMed
-
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

