Organismal carbohydrate and lipid homeostasis
- PMID: 22550228
- PMCID: PMC3331699
- DOI: 10.1101/cshperspect.a006031
Organismal carbohydrate and lipid homeostasis
Abstract
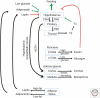
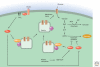
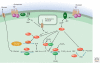
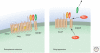
All living organisms maintain a high ATP:ADP ratio to drive energy-requiring processes. They therefore need mechanisms to maintain energy balance at the cellular level. In addition, multicellular eukaryotes have assigned the task of storing energy to specialized cells such as adipocytes, and therefore also need a means of intercellular communication to signal the needs of individual tissues and to maintain overall energy balance at the whole body level. Such signaling allows animals to survive periods of fasting or starvation when food is not available and is mainly achieved by hormonal and nervous communication. Insulin, adipokines, epinephrine, and other agonists thus stimulate pathways that regulate the activities of key enzymes involved in control of metabolism to integrate organismal carbohydrate and lipid metabolism. Overnutrition can dysregulate these pathways and have damaging consequences, causing insulin resistance and type 2 diabetes.
Figures


References
-
- Berggreen C, Gormand A, Omar B, Degerman E, Goransson O 2009. Protein kinase B activity is required for the effects of insulin on lipid metabolism in adipocytes. Am J Physiol Endocrinol Metab 296: E635–E646 - PubMed
-
- Bonen A, Han XX, Habets DD, Febbraio M, Glatz JF, Luiken JJ 2007. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am J Physiol Endocrinol Metab 292: E1740–E1749 - PubMed
-
- Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA, Voshol PJ, Jensen J, Sakamoto K 2010. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle. Cell Metab 12: 456–466 - PubMed
-
- Brasaemle DL 2007. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J Lipid Res 48: 2547–2559 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
