Autophagy in alcohol-induced liver diseases
- PMID: 22551004
- PMCID: PMC3653416
- DOI: 10.1111/j.1530-0277.2012.01742.x
Autophagy in alcohol-induced liver diseases
Abstract
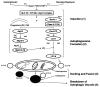
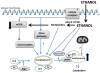
Alcohol is the most abused substance worldwide and a significant source of liver injury; the mechanisms of alcohol-induced liver disease are not fully understood. Significant cellular toxicity and impairment of protein synthesis and degradation occur in alcohol-exposed liver cells, along with changes in energy balance and modified responses to pathogens. Autophagy is the process of cellular catabolism through the lysosomal-dependent machinery, which maintains a balance among protein synthesis, degradation, and recycling of self. Autophagy is part of normal homeostasis and it can be triggered by multiple factors that threaten cell integrity, including starvation, toxins, or pathogens. Multiple factors regulate autophagy; survival and preservation of cellular integrity at the expense of inadequately folded proteins and damaged high-energy generating intracellular organelles are prominent targets of autophagy in pathological conditions. Coincidentally, inadequately folded proteins accumulate and high-energy generating intracellular organelles, such as mitochondria, are damaged by alcohol abuse; these alcohol-induced pathological findings prompted investigation of the role of autophagy in the pathogenesis of alcohol-induced liver damage. Our review summarizes the current knowledge about the role and implications of autophagy in alcohol-induced liver disease.
Copyright © 2012 by the Research Society on Alcoholism.
Figures

References
-
- Baraona E, Leo MA, Borowsky SA, Lieber CS. Alcoholic hepatomegaly: accumulation of protein in the liver. Science. 1975;190(4216):794–5. - PubMed
-
- Bardag-Gorce F, Li J, French BA, French SW. The effect of ethanol-induced CYP2E1 on proteasome activity: the role of 4-hydroxynonenal. Exp Mol Pathol. 2005;78(2):109–15. - PubMed
-
- Bergamini E, Cavallini G, Donati A, Gori Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed Pharmacother. 2003;57(5-6):203–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21AA016571/AA/NIAAA NIH HHS/United States
- P20 RR016475/RR/NCRR NIH HHS/United States
- R21AA017421/AA/NIAAA NIH HHS/United States
- R01AA02518/AA/NIAAA NIH HHS/United States
- R01 CA119079/CA/NCI NIH HHS/United States
- C06RR015455/RR/NCRR NIH HHS/United States
- P20 GM103418/GM/NIGMS NIH HHS/United States
- R01 AA020518/AA/NIAAA NIH HHS/United States
- R01DK073336/DK/NIDDK NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- P20RR021940/RR/NCRR NIH HHS/United States
- R01 AA017212/AA/NIAAA NIH HHS/United States
- R01CA119079/CA/NCI NIH HHS/United States
- P01 DK059340/DK/NIDDK NIH HHS/United States
- P20 RR021940/RR/NCRR NIH HHS/United States
- P20RR016475/RR/NCRR NIH HHS/United States
- R01DK37034/DK/NIDDK NIH HHS/United States
- P01DK59340/DK/NIDDK NIH HHS/United States
- R37 AA020518/AA/NIAAA NIH HHS/United States
- R01AA017212/AA/NIAAA NIH HHS/United States
- R21 AA017421/AA/NIAAA NIH HHS/United States
- R01 DK037034/DK/NIDDK NIH HHS/United States
- R01 DK073336/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

