Efficacy of an immunotoxin to folate receptor beta in the intra-articular treatment of antigen-induced arthritis
- PMID: 22551402
- PMCID: PMC3446483
- DOI: 10.1186/ar3831
Efficacy of an immunotoxin to folate receptor beta in the intra-articular treatment of antigen-induced arthritis
Abstract
Introduction: We previously demonstrated that synovial sublining macrophages express folate receptor beta (FRβ). The aim of this study was to evaluate the efficacy of intra-articular administration of a recombinant immunotoxin to FRβ for treating rat antigen-induced arthritis.
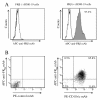
Methods: A monoclonal antibody (mAb) to rat FRβ was produced by immunizing mice with B300-19 cells (murine pre-B cells) transfected with the rat FRβ gene. Recombinant immunotoxin was prepared by conjugating the Fv portion of the anti-rat FRβ mAb heavy chain with a truncated Pseudomonas exotoxin A and the Fv portion of the anti-rat FRβ mAb light chain. Antigen-induced arthritis was induced through intra-articular injection of methylated bovine serum albumin (mBSA) after two subcutaneous injections of mBSA and complete Freund's adjuvant. Immunotoxin was intra-articularly injected into the arthritis joint every other day for seven days after arthritis onset. Joint swelling was measured and histological scores of inflammation, synovial thickness, cartilage, and bone destruction were determined. Immunohistochemistry was performed to detect osteoclast and osteoclast precursor FRβ-expressing macrophages and cathepsin K-positive cells on day 21.
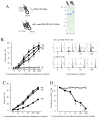
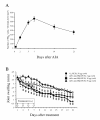
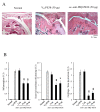
Results: Intra-articular administration of the immunotoxin attenuated joint swelling (61% suppression; P < 0.01 compared to the control on day 21) and improved histological findings, particularly cartilage and bone destruction (scores of rats treated with control versus the immunotoxin: 2.2 versus 0.5; P < 0.01), by reducing the number of FRβ-expressing macrophages and cathepsin K-positive cells.
Conclusions: Intra-articular administration of an immunotoxin to FRβ is effective for improving rat antigen-induced arthritis.
Figures


References
-
- McHugh KP, Shen Z, Crotti TN, Flannery MR, O'Sullivan RP, Purdue PE, Goldring SR. The role of cell-substrate interaction in regulating osteoclast activation: potential implications in targeting bone loss in rheumatoid arthritis. Ann Rheum Dis. 2010;69(Suppl 1):i83–85. doi: 10.1136/ard.2009.120188. - DOI - PubMed
-
- Bresnihan B, Pontifex E, Thurlings RM, Vinkenoog M, El-Gabalawy H, Fearon U, Fitzgerald O, Gerlag DM, Rooney T, van de Sande MG, Veale D, Vos K, Tak PP. Synovial tissue sublining CD68 expression is a biomarker of therapeutic response in rheumatoid arthritis clinical trials: consistency across centers. J Rheumatol. 2009;36:1800–1802. doi: 10.3899/jrheum.090348. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

