Decreased bone mineralization in children with Noonan syndrome: another consequence of dysregulated RAS MAPKinase pathway?
- PMID: 22551697
- PMCID: PMC3356458
- DOI: 10.1016/j.ymgme.2012.04.003
Decreased bone mineralization in children with Noonan syndrome: another consequence of dysregulated RAS MAPKinase pathway?
Abstract
Introduction: Noonan syndrome (NS) is a disorder of RAS- mitogen activated protein kinase (MAPK) pathway with clinical features of skeletal dysplasia. This pathway is essential for regulation of cell differentiation and growth including bone homeostasis. Currently, limited information exists regarding bone mineralization in NS.
Materials and methods: Using dual-energy X-ray absorptiometry (DXA), bone mineralization was evaluated in 12 subjects (mean age 8.7 years) with clinical features of NS. All subjects underwent genetic testing which showed mutations in PTPN11 gene (N=8) and SOS1 gene (N=1). In a subgroup of subjects with low bone mass, indices of calcium-phosphate metabolism and bone turnover were obtained.
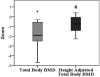
Results: 50% of subjects had low bone mass as measured by DXA. Z-scores for bone mineral content (BMC) were calculated based on age, gender, height, and ethnicity. Mean BMC z-score was marginally decreased at -0.89 {95% CI -2.01 to 0.23; p=0.1}. Mean total body bone mineral density (BMD) z-score was significantly reduced at -1.87 {95% CI -2.73 to -1.0; p=0.001}. Mean height percentile was close to - 2 SD for this cohort, thus total body BMD z-scores were recalculated, adjusting for height age. Adjusted mean total body BMD z-score was less reduced but still significant at -0.82 {95% CI -1.39 to -0.25; p=0.009}. Biochemical evaluation for bone turnover was unremarkable except serum IGF-I and IGF-BP3 levels which were low-normal for age.
Discussion: Children with NS have a significantly lower total body BMD compared to age, gender, ethnicity and height matched controls. In addition, total BMC appears to trend lower in children with NS compared to controls. We conclude that the metabolic bone disease present resulted from a subtle variation in the interplay of osteoclast and osteoblast activity, without clear abnormalities being defined in the metabolism of either. Clinical significance of this finding needs to be validated by larger longitudinal studies. Also, histomorphometric analysis of bone tissue from NS patients and mouse model of NS may further elucidate the relationship between the RAS-MAPK pathway and skeletal homeostasis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Gelb BD, Tartaglia M. Noonan syndrome and related disorders: dysregulated RAS-mitogen activated protein kinase signal transduction. Human molecular genetics. 2006;15:R220–226. Spec No 2. - PubMed
-
- Carta C, Pantaleoni F, Bocchinfuso G, Stella L, Vasta I, Sarkozy A, Digilio C, Palleschi A, Pizzuti A, Grammatico P, Zampino G, Dallapiccola B, Gelb BD, Tartaglia M. Germline missense mutations affecting KRAS Isoform B are associated with a severe Noonan syndrome phenotype. American journal of human genetics. 2006;79:129–135. - PMC - PubMed
-
- Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, Bollag G, van der Burgt I, Musante L, Kalscheuer V, Wehner LE, Nguyen H, West B, Zhang KY, Sistermans E, Rauch A, Niemeyer CM, Shannon K, Kratz CP. Germline KRAS mutations cause Noonan syndrome. Nature genetics. 2006;38:331–336. - PubMed
-
- Zenker M, Horn D, Wieczorek D, Allanson J, Pauli S, van der Burgt I, Doerr HG, Gaspar H, Hofbeck M, Gillessen-Kaesbach G, Koch A, Meinecke P, Mundlos S, Nowka A, Rauch A, Reif S, von Schnakenburg C, Seidel H, Wehner LE, Zweier C, Bauhuber S, Matejas V, Kratz CP, Thomas C, Kutsche K. SOS1 is the second most common Noonan gene but plays no major role in cardio-facio-cutaneous syndrome. Journal of medical genetics. 2007;44:651–656. - PMC - PubMed
-
- Tartaglia M, Martinelli S, Cazzaniga G, Cordeddu V, Iavarone I, Spinelli M, Palmi C, Carta C, Pession A, Arico M, Masera G, Basso G, Sorcini M, Gelb BD, Biondi A. Genetic evidence for lineage-related and differentiation stage-related contribution of somatic PTPN11 mutations to leukemogenesis in childhood acute leukemia. Blood. 2004;104:307–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous