Inhibition of cell surface expression of endothelial adhesion molecules by ursolic acid prevents intimal hyperplasia of venous bypass grafts in rats
- PMID: 22551965
- PMCID: PMC3523388
- DOI: 10.1093/ejcts/ezs128
Inhibition of cell surface expression of endothelial adhesion molecules by ursolic acid prevents intimal hyperplasia of venous bypass grafts in rats
Abstract
Objectives: Despite rapid progress in surgical techniques, there is still a significant lack of surgery-supportive pharmacological treatments. The aim of this study was to test the hypothesis that ursolic acid (UA) may prevent intimal hyperplasia of venous bypass grafts.
Methods: The hypothesis was tested by means of primary cell isolation and culture followed by real-time polymerase chain reaction, western blotting, fluorescence microscopy and fluorescence-activated cell sorting analyses, as well as an in vivo rat model for intimal hyperplasia of venous bypass grafts and immunohistochemistry and histochemistry.
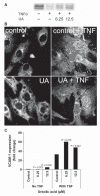
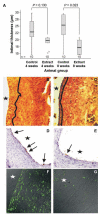
Results: The local application of UA significantly inhibited intimal hyperplasia in vivo (intimal thickness control: 25 µm, UA group: 18 µM-8 weeks after surgery). The UA treatment of grafts significantly resulted in reduced endothelial vascular cell adhesion molecule-1 (VCAM-1) expression, reduced infiltration of the grafts vessel wall by CD45-positive cells and increased smooth muscle cell (SMC) death. In in vitro condition, it could be shown that UA inhibits VCAM-1 expression downstream of NFκB and is likely to interfere with VCAM-1 protein synthesis in endothelial cells. Quantification of cell death in vascular smooth muscle cells treated with UA indicated that UA is a potent inducer of SMC apoptosis.
Conclusions: Our results suggest that UA-mediated inhibition of endothelial VCAM-1 expression reduces the infiltration of venous bypass grafts by CD45-positive cells and inhibits intimal hyperplasia. Apoptosis induction in SMCs may be another method in which UA reduces intimal thickening. UA may constitute a surgery-supportive pharmacon that reduces intimal hyperplasia of vein grafts.
Figures


References
-
- Crook MF, Newby AC, Southgate KM. Expression of intercellular adhesion molecules in human saphenous veins: effects of inflammatory cytokines and neointima formation in culture. Atherosclerosis. 2000;150:33–41. - PubMed
-
- Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7:328–31. - PubMed
-
- Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, et al. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114:1435–140. - PubMed
-
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

