Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide
- PMID: 22552008
- PMCID: PMC3496085
- DOI: 10.1038/leu.2012.119
Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide
Erratum in
- Leukemia. 2012 Nov;26(11):2445
Abstract
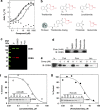
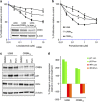
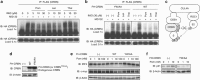
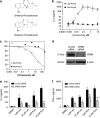
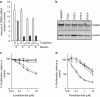
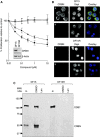
Thalidomide and the immunomodulatory drug, lenalidomide, are therapeutically active in hematological malignancies. The ubiquitously expressed E3 ligase protein cereblon (CRBN) has been identified as the primary teratogenic target of thalidomide. Our studies demonstrate that thalidomide, lenalidomide and another immunomodulatory drug, pomalidomide, bound endogenous CRBN and recombinant CRBN-DNA damage binding protein-1 (DDB1) complexes. CRBN mediated antiproliferative activities of lenalidomide and pomalidomide in myeloma cells, as well as lenalidomide- and pomalidomide-induced cytokine production in T cells. Lenalidomide and pomalidomide inhibited autoubiquitination of CRBN in HEK293T cells expressing thalidomide-binding competent wild-type CRBN, but not thalidomide-binding defective CRBN(YW/AA). Overexpression of CRBN wild-type protein, but not CRBN(YW/AA) mutant protein, in KMS12 myeloma cells, amplified pomalidomide-mediated reductions in c-myc and IRF4 expression and increases in p21(WAF-1) expression. Long-term selection for lenalidomide resistance in H929 myeloma cell lines was accompanied by a reduction in CRBN, while in DF15R myeloma cells resistant to both pomalidomide and lenalidomide, CRBN protein was undetectable. Our biophysical, biochemical and gene silencing studies show that CRBN is a proximate, therapeutically important molecular target of lenalidomide and pomalidomide.
Figures
References
-
- Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. - PubMed
-
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. - PubMed
-
- Zeldis JB, Knight R, Hussein M, Chopra R, Muller G. A review of the history, properties, and use of the immunomodulatory compound lenalidomide. Ann N Y Acad Sci. 2011;1222:76–82. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Research Materials

