Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques
- PMID: 22552291
- PMCID: PMC3533365
- DOI: 10.1158/0008-5472.CAN-12-0128
Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques
Abstract
The blood-brain barrier (BBB) prevents entry of most drugs into the brain and is a major hurdle to the use of drugs for brain tumors and other central nervous system disorders. Work in small animals has shown that ultrasound combined with an intravenously circulating microbubble agent can temporarily permeabilize the BBB. Here, we evaluated whether this targeted drug delivery method can be applied safely, reliably, and in a controlled manner on rhesus macaques using a focused ultrasound system. We identified a clear safety window during which BBB disruption could be produced without evident tissue damage, and the acoustic pressure amplitude where the probability for BBB disruption was 50% and was found to be half of the value that would produce tissue damage. Acoustic emission measurements seem promising for predicting BBB disruption and damage. In addition, we conducted repeated BBB disruption to central visual field targets over several weeks in animals trained to conduct complex visual acuity tasks. All animals recovered from each session without behavioral deficits, visual deficits, or loss in visual acuity. Together, our findings show that BBB disruption can be reliably and repeatedly produced without evident histologic or functional damage in a clinically relevant animal model using a clinical device. These results therefore support clinical testing of this noninvasive-targeted drug delivery method.
Conflict of interest statement
Figures
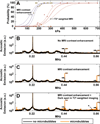
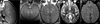
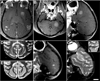
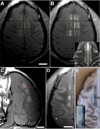


References
-
- Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2(3):106–113. PMID: 8796867. - PubMed
-
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6(2):252–273. PMID: 12935438. - PubMed
-
- Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–1099. - PubMed
-
- Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. PMID: 17198973. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

